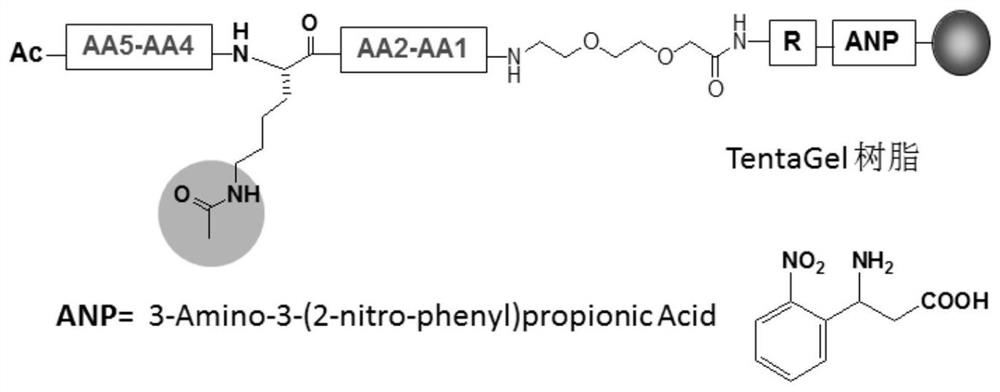
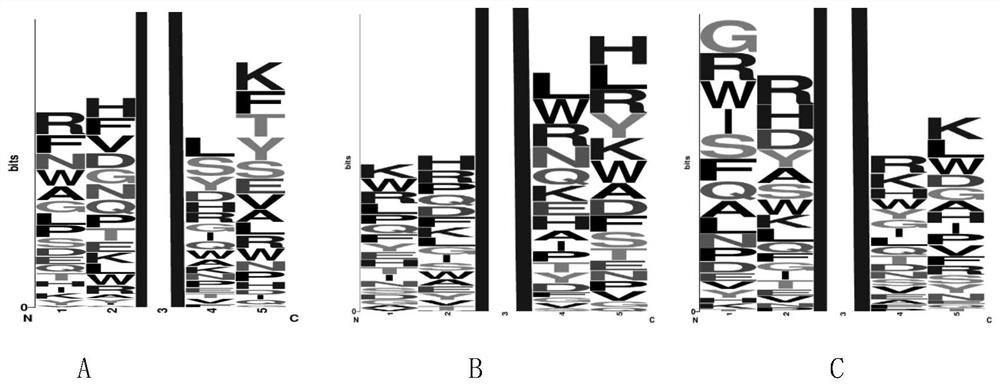
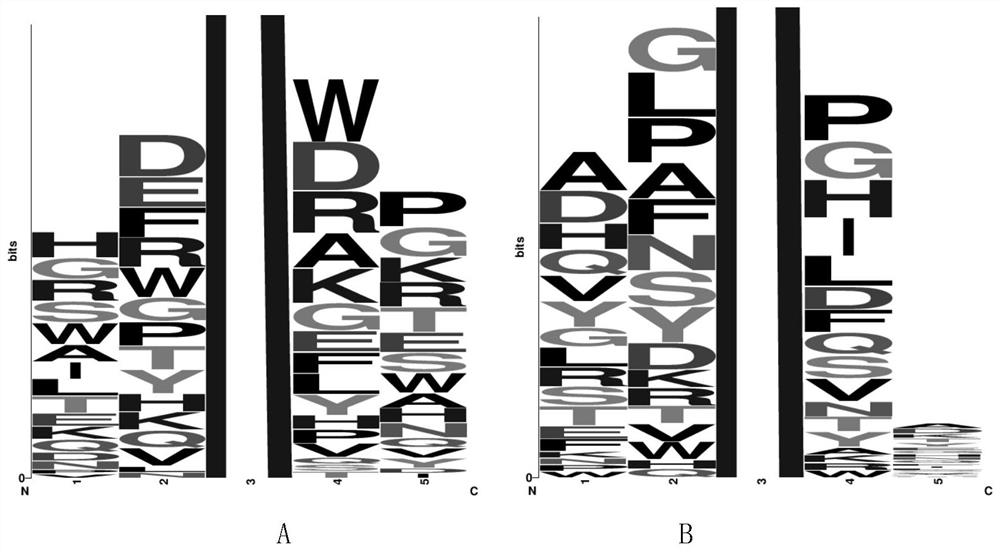
BRD4 protein targeting anti-tumor polypeptide and application thereof
A technology of targeting and peptide beads, which can be used in anti-tumor drugs, peptides, peptide libraries, etc., and can solve the problems of toxic side effects, poor selectivity, and low specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Embodiment 1 targeting BRD4-BD1 domain polypeptide
[0088] Targeting BRD4-BD1 domain polypeptides, the core amino acid sequences are: Ac-KR-K(Ac)-VS, Ac-TG-K(Ac)-WS, Ac-IQ-K(Ac)-RP, Ac - LA-K(Ac)-SF, Ac-DP-K(Ac)-IT and Ac-AD-K(Ac)-DG.
[0089] Table 1 The polypeptide sequence targeting BRD4-BD1 domain
[0090] Peptide number amino acid sequence of polypeptide B1-1 Ac-KR-K(Ac)-VS (SEQ ID NO.: 1) B1-2 Ac-TG-K(Ac)-WS (SEQ ID NO.:2) B1-5 Ac-IQ-K(Ac)-RP (SEQ ID NO.: 3) B1-6 Ac-LA-K(Ac)-SF (SEQ ID NO.:4) B1-7 Ac-DP-K(Ac)-IT (SEQ ID NO.:5) B1-8 Ac-AD-K(Ac)-DG (SEQ ID NO.:6)
Embodiment 2
[0091] Embodiment 2 targeting BRD4-BD2 domain polypeptide
[0092] Targeting BRD4-BD2 structure and polypeptide, its core amino acid sequences are: GD-K(Ac)-LY, WR-K(Ac)-PD, IT-K(Ac)-NL, YK-K(Ac)- PY, GV-K(Ac)-SR, RH-K(Ac)-LK.
[0093] The polypeptide contains acetylated lysine K (Ac), and except the third amino acid, the amino acids at other positions are L-type natural amino acids. Concrete operation is with embodiment 1.
[0094] Table 2 The polypeptide sequence targeting the BRD4-BD2 domain
[0095] Peptide number amino acid sequence of polypeptide B2-1 Ac-WR-K(Ac)-PD (SEQ ID NO.7) B2-2 Ac-IT-K(Ac)-NL (SEQ ID NO.8) B2-3 Ac-YK-K(Ac)-PY (SEQ ID NO.9) B2-4 Ac-RH-K(Ac)-LK (SEQ ID NO.10) B2-5 Ac-GD-K(Ac)-LY (SEQ ID NO.11) B2-6 Ac-GV-K(Ac)-SR (SEQ ID NO.12)
Embodiment 3
[0096] Example 3 Screening of acetylated lysine polypeptides
[0097] (1) Synthesis of acetylated lysine peptide library
[0098] The present invention selects 20 kinds of L-type natural amino acids to form a pentapeptide compound library; the compound library is a pentapeptide sequence in which the third amino acid is acetylated lysine, and the amino acids at both ends are arranged and combined with 20 kinds of natural amino acids, expressed as NH 2 -AA 5 -AA 4 -K(Ac)-AA 2 -AA 1 -CONH 2 , up to 20 types of peptide sequences 4 kind. The peptide compound library was synthesized by an automatic peptide synthesizer, and then the amino acids with higher frequency at each site were selected after preliminary screening and mass spectrometry sequencing by COPAS, and the peptide compound library was further constructed. The peptide compound library is screened to obtain and optimize the peptide sequence with higher affinity. The peptide library was synthesized using an automat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com