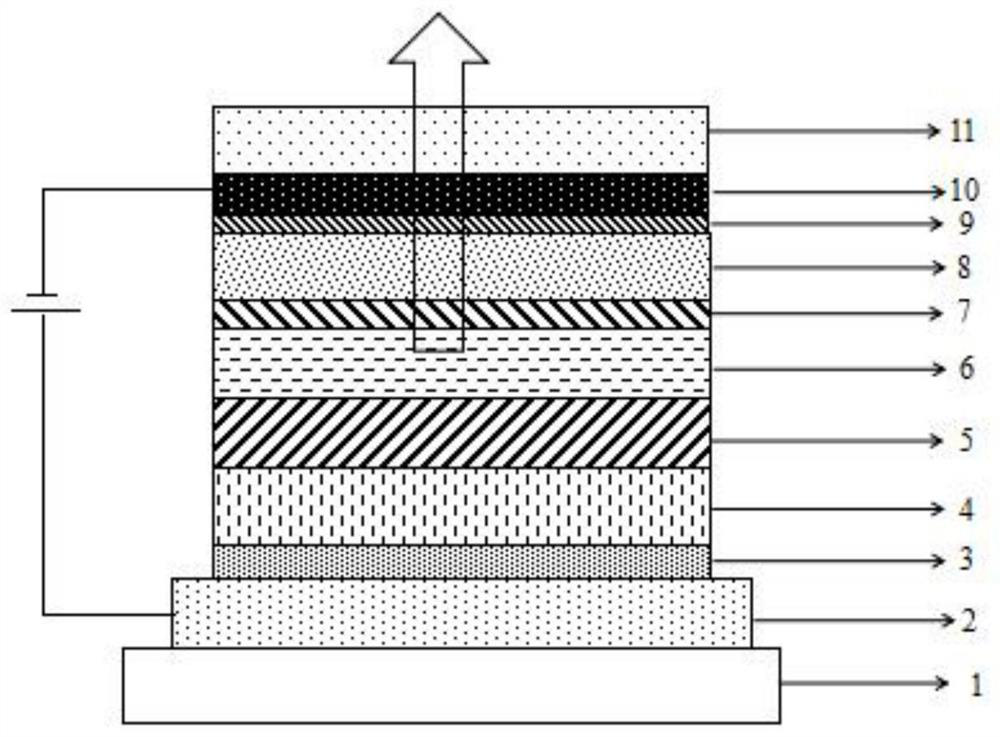
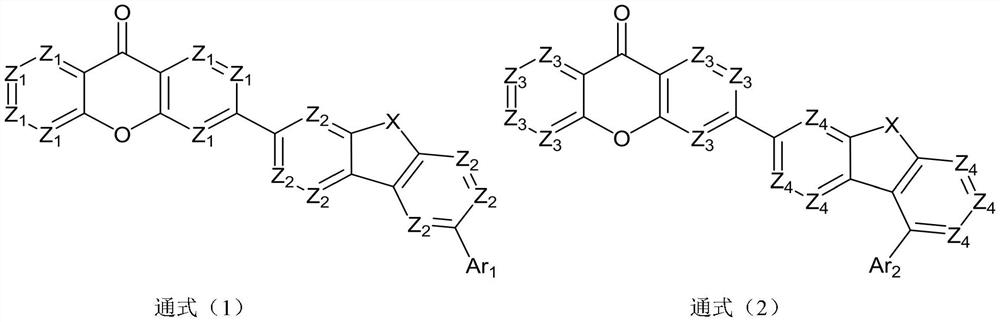
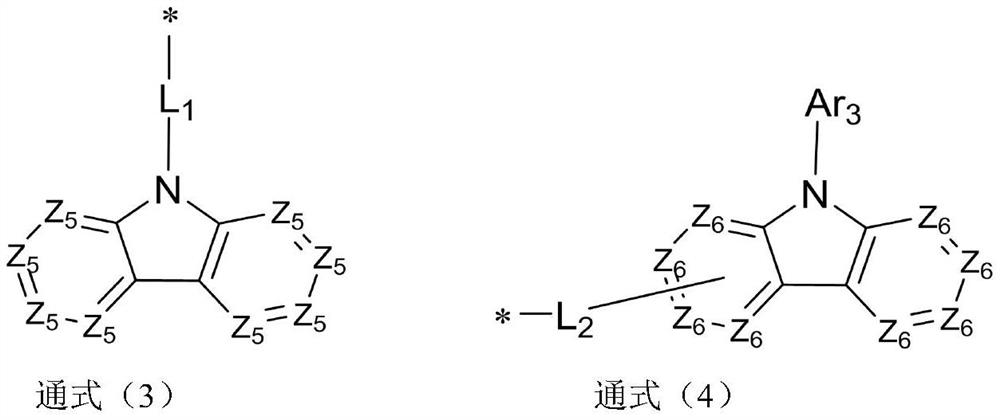
Compound taking dibenzo five-membered heterocyclic ring as core and application thereof
A five-membered heterocycle and compound technology, applied in the field of semiconductors, can solve the problems of device efficiency and lifespan to be improved, short lifespan, inability to balance electron transport and hole transport in the light-emitting layer, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Embodiment 1. Preparation of compound 1:
[0090]
[0091] (1) In a three-necked flask, under the protection of nitrogen, weigh 0.01mol of raw material A-1 and dissolve it in tetrahydrofuran, cool to -78°C, and then add 8ml of 1.6mol / L n-butyllithium tetrahydrofuran solution to the reaction system , after reacting for 3 hours at -78°C, add 0.013mol triisopropyl borate, react for 2h, then raise the reaction system to 0°C, add 10ml 2mol / L hydrochloric acid solution, stir for 3h, the reaction is complete, add ether to extract, the extract Add anhydrous magnesium sulfate to dry, spin evaporate, and recrystallize with ethanol solvent to obtain intermediate I-1;
[0092] (2) In the three-necked flask, under the protection of nitrogen, add 0.01mol intermediate I-1, 0.012mol raw material B-1, dissolve with mixed solvent (90ml toluene, 45ml ethanol), and then add 1×10 -4 mol Pd(PPh 3 ) 4 , 0.03molK 2 CO 3 Aqueous solution (2M), heating and reflux reaction for 15 hours, s...
Embodiment 4
[0094] Example 4. Preparation of compound 33:
[0095]
[0096] (1) In a three-necked flask, under the protection of nitrogen, weigh 0.01mol of raw material A-2 and dissolve it in tetrahydrofuran, cool to -78°C, and then add 8ml of 1.6mol / L n-butyllithium tetrahydrofuran solution to the reaction system , after reacting for 3 hours at -78°C, add 0.013mol triisopropyl borate, react for 2h, then raise the reaction system to 0°C, add 10ml 2mol / L hydrochloric acid solution, stir for 3h, the reaction is complete, add ether to extract, the extract Add anhydrous magnesium sulfate to dry, spin evaporate, and recrystallize with ethanol solvent to obtain intermediate I-2;
[0097] (2) In the three-neck flask, under the protection of nitrogen, add 0.01mol intermediate I-2, 0.012mol raw material B-1, dissolve with mixed solvent (90ml toluene, 45ml ethanol), and then add 1×10 -4 mol Pd(PPh 3 ) 4 , 0.03molK 2 CO 3 Aqueous solution (2M), heating and reflux reaction for 15 hours, sampl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com