A kind of medicine for treating polycystic ovary syndrome and preparation method thereof
A technology of drugs and compounds, applied in the field of medicinal chemistry, can solve problems such as incurable diseases and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
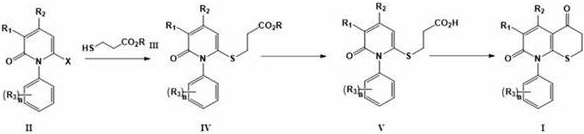
[0045] The present invention provides a kind of preparation method of formula I compound, described method comprises the following steps:
[0046]
[0047] The compound of formula II is reacted with the compound of formula III to generate the compound of formula IV, and after being hydrolyzed to generate the acid of formula V, the compound of formula I is cyclized.
[0048] Wherein, X represents halogen, preferably chlorine or bromine; R represents C1-C6 alkyl, preferably methyl or ethyl; R 1 -R 3 , n are as defined in the present invention.
[0049]In a preferred embodiment, the reaction of the compound of formula II and the compound of formula III is carried out in the presence of a base, the base is selected from organic bases or inorganic bases, the organic base is selected from triethylamine, pyridine, and the inorganic base Selected from sodium carbonate, potassium carbonate, cesium carbonate, sodium hydroxide, potassium hydroxide; the molar ratio of the compound of...
specific Embodiment approach
[0066] Hereinafter, the present invention is described in more detail to facilitate understanding of the present invention.
[0067] Those skilled in the art will recognize that the chemical reactions described herein can be used to suitably prepare many other compounds of the invention and that other methods for preparing the compounds of the invention are considered to be within the scope of the invention Inside. For example, the synthesis of those non-exemplified compounds according to the present invention can be successfully accomplished by those skilled in the art through modification methods, such as appropriate protection of interfering groups, by using other known reagents in addition to those described in the present invention, or by incorporating Reaction conditions with some routine modifications. In addition, reactions disclosed herein or known reaction conditions are also recognized to be applicable to the preparation of other compounds of this invention.
Embodiment 1
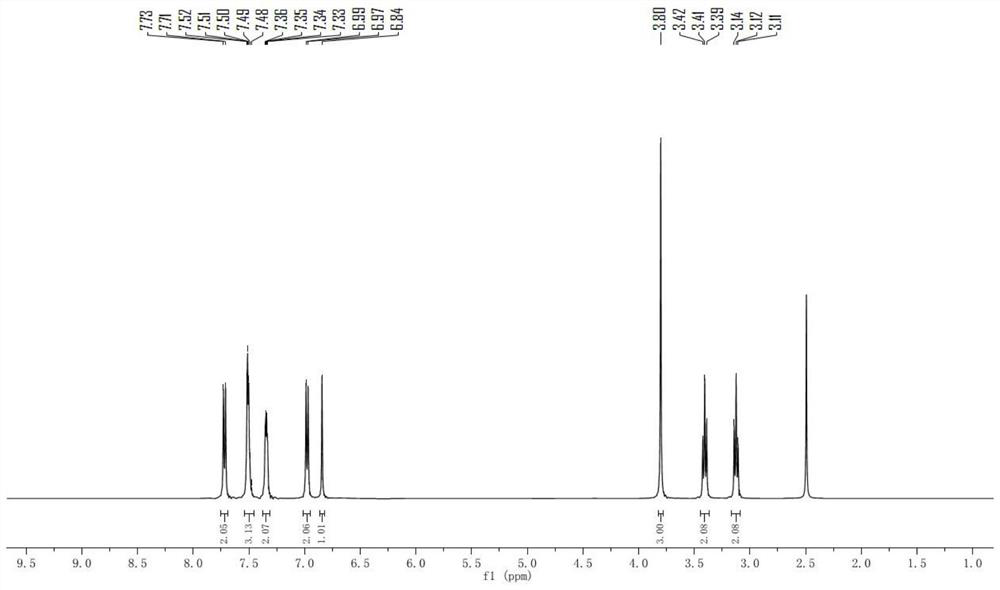
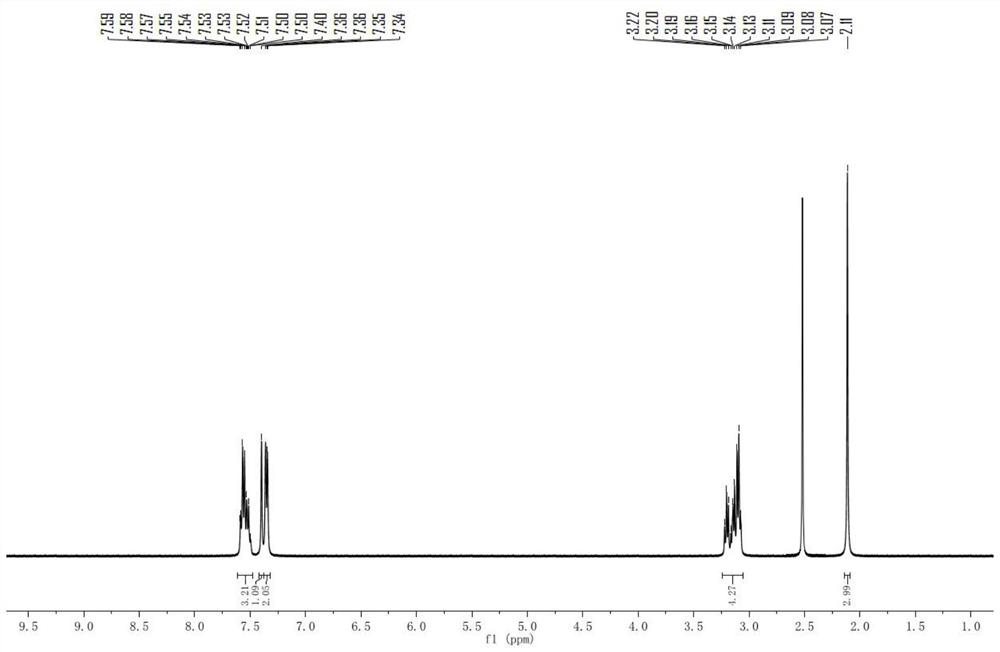
[0068] Example 1: 5-(4-methoxyphenyl)-8-phenyl-2,3-dihydro-4H-thiopyran[2,3-b]pyridine-4,7(8H)-dione (Compound 1) Preparation
[0069]
[0070] Add ethyl 3-mercaptopropionate (20mmol), 6g of anhydrous potassium carbonate, and 80mL of N,N-dimethylformamide into a flask equipped with a magnetic stirrer and a reflux condenser, and stir at room temperature for 20 minutes under argon protection , with a constant pressure dropping funnel, 6-chloro-4-(4-methoxyphenyl)-1-phenylpyridin-2(1H)-one (20mmol) in 20mLN,N-dimethylformamide solution Slowly add it dropwise into the reaction system, slowly raise the reaction temperature to 85°C, and react for 12 hours. After the reaction is complete, cool to room temperature, pour the reaction solution into an appropriate amount of ice water, stir for 5 minutes, then extract with ethyl acetate as the extractant 3 times, 100 mL each time, combine the organic phases, wash 2 times with distilled water and brine respectively, each time 150mL, d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com