Proteolysis targeting virus, live vaccine thereof, preparation method of proteolysis targeting virus, preparation method of live vaccine, application of proteolysis targeting virus, and application of preparation method
A proteolytic and viral technology, applied in the biological field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0155] Example 1 Establishment of stable mammalian cell lines HEK293-TEVp and MDCK-TEVp capable of stably expressing TEVp.
[0156] The mammalian cell line stably expressing TEVp was commissioned by Beijing CorreGene Biotechnology Co., Ltd. (CorreGene).
[0157] (1) Construction of TEVp overexpression lentiviral vector:
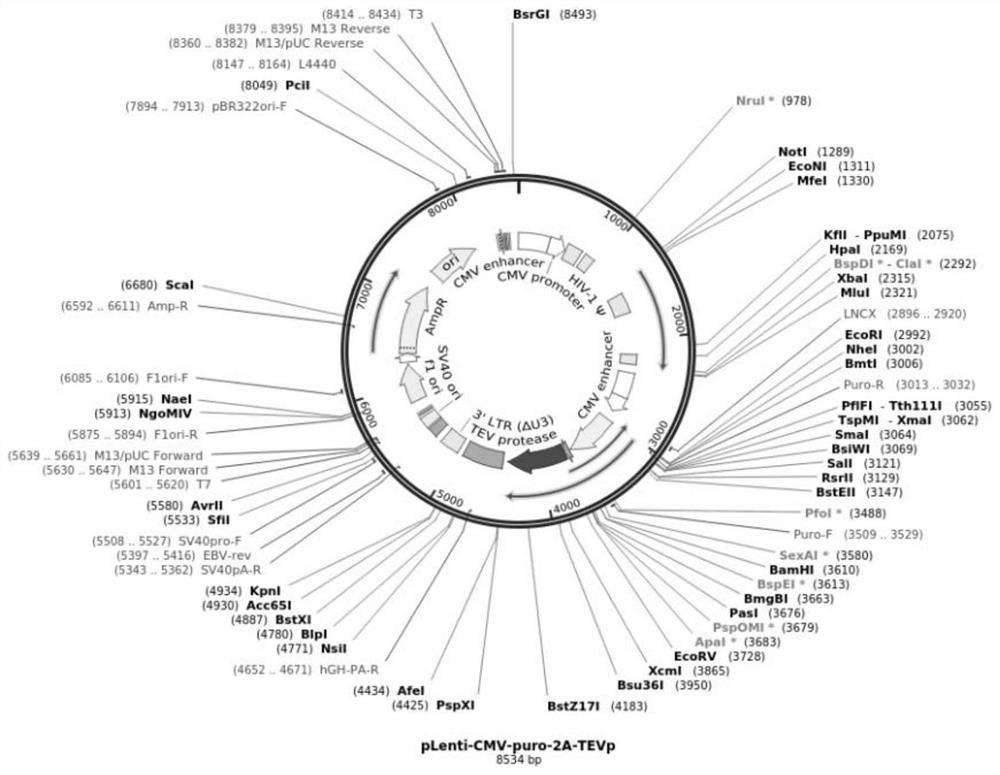
[0158] TEVp overexpression lentiviral vector map such as figure 1 shown. The lentiviral vector is puromycin resistant.
[0159] (2) Purification and quantification of lentiviral packaging
[0160] ① Cell inoculation on the 0th day: 293T was inoculated in a 10cm / 15cm culture dish (the inoculum number is determined by the expected amount of virus, 1×10^8 / 15cm culture dish), control the inoculation density, and grow to 80% confluence the next day;
[0161] ②Plasmid transfection on day 1: Use the TEVp overexpression lentiviral vector described in (1), psPAX2 plasmid (this plasmid can be obtained from addgene) and pVSVG plasmid (this plasmid can be obtained fr...
Embodiment 2
[0194] Example 2: Construction of a gene vector for influenza virus WSN comprising a cleavable proteolytic targeting molecule.
[0195] (1) Rescue the acquisition of the plasmid of wild-type influenza virus WSN:
[0196] According to the gene sequence of influenza virus A / WSN / 1933 published by pubmed
[0197] https: / / www.ncbi.nlm.nih.gov / nuccore / ? term=WSN+PB2 ;
[0198] https: / / www.ncbi.nlm.nih.gov / nuccore / ? term=WSN+PB1 ;
[0199] https: / / www.ncbi.nlm.nih.gov / nuccore / ? term=WSN+PA ;
[0200] https: / / www.ncbi.nlm.nih.gov / nuccore / ? term=WSN+HA ;
[0201] https: / / www.ncbi.nlm.nih.gov / nuccore / ? term=WSN+NA ;
[0202] https: / / www.ncbi.nlm.nih.gov / nuccore / ? term=WSN+NP;
[0203] https: / / www.ncbi.nlm.nih.gov / nuccore / ? term=WSN+M ;
[0204] https: / / www.ncbi.nlm.nih.gov / nuccore / ? term=WSN+NS, the genes of each gene segment of the influenza virus are obtained through whole gene synthesis. Then they were connected to pHH21, pCDNA3(neo), and pcAAGGS / MCS ve...
Embodiment 3
[0237] Example 3: Rescue of PROTAC influenza virus modified by site-directed mutagenesis
[0238] According to the normal influenza virus rescue method, the 12 plasmids used for influenza virus rescue were co-transfected into a stable cell line, and the corresponding plasmids among the 12 plasmids were replaced with the plasmids transformed by site-directed mutagenesis in Example 2. Corresponding to each well of the six-well plate, add 0.2 μg of each plasmid. After transfection, observe the pathological changes of the cells, and screen out insertion sites that can rescue the virus and are dependent on TEVp, proteolysis targeting molecules, linkers that can be cleaved by TEVp, and combinations thereof. The screened strains were named according to the protein and the introduced cleavable proteolytic targeting molecule.
[0239] As an example, after introducing the cleavable proteolytic targeting molecule TEVcs+PROTAC-1 on the Ben3 pPolI-WSN-PA plasmid, this plasmid was combin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com