A negative low temperature curable photosensitive resin composition
A technology of photosensitive resin and composition, applied in optics, optomechanical equipment, photosensitive materials for optomechanical equipment, etc., to achieve the effects of excellent chemical resistance, excellent reliability, and low temperature process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
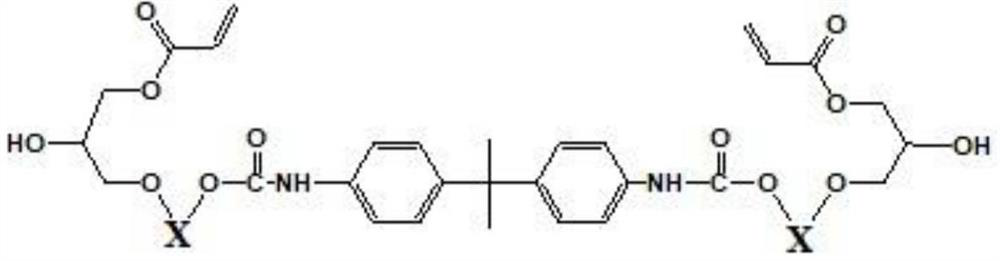
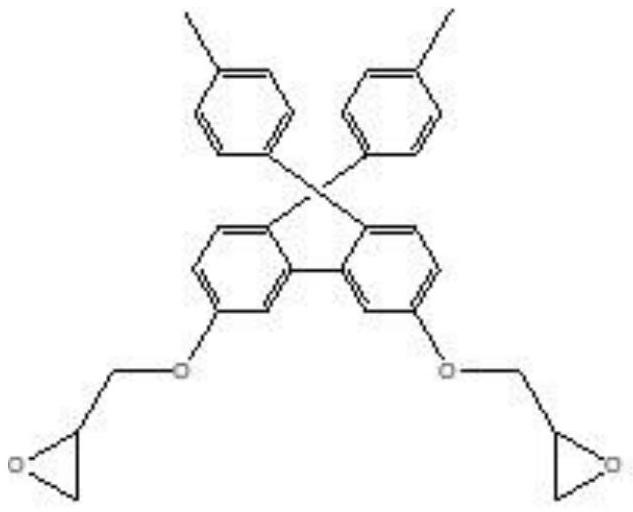
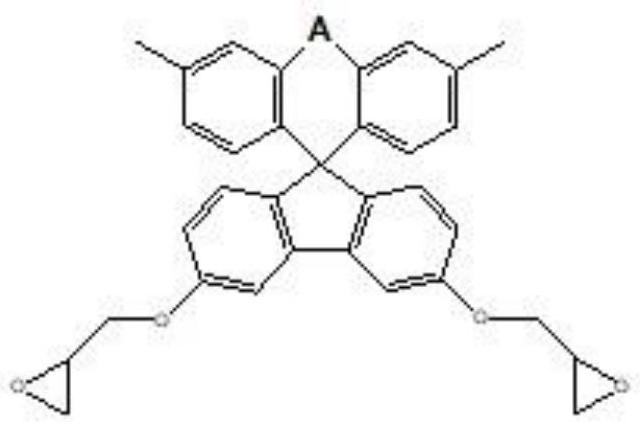
[0078] After preparing a flask equipped with a mechanical stirrer, a thermometer, and a cooling jacket, 240 g of propylene glycol monomethyl ether and 100 g of bisphenol fluorene represented by Chemical Formula 1-1 were added under a nitrogen stream, and heated to 40° C. while stirring. After adding 0.288 g of dibutyltin dilaurate to the reaction liquid, the temperature of the reactor was raised to 65°C. While continuing to stir the reaction solution, 36.98 g of 4,4'-diphenylmethane diisocyanate was slowly added separately, and the absorption spectrum of the isocyanate group (2280 cm -1 ) disappeared, and 315.2 g of epichlorohydrin was added to the reaction liquid, followed by heating to 100° C. and stirring for 1 hour. To this reaction liquid, 33.8 g of methacrylic acid was added, and the temperature was raised to 110° C. to make it react for 3 hours. Thereafter, it was dissolved in tetrahydrofuran (THF), and after obtaining a precipitate with water, it was dissolved in dich...
Synthetic example 2
[0080] After preparing a flask equipped with a mechanical stirrer, a thermometer, and a cooling jacket, 240 g of propylene glycol monomethyl ether and 100 g of bisphenol fluorene represented by Chemical Formula 1-1 were added under a nitrogen stream, and heated to 40° C. while stirring. After adding 0.288 g of dibutyltin dilaurate to the reaction liquid, the temperature of the reactor was raised to 65°C. While continuing to stir the reaction solution, 36.98 g of 4,4'-diphenylmethane diisocyanate was slowly added separately, and the absorption spectrum of the isocyanate group (2280 cm -1 ) disappeared, and 315.2 g of epichlorohydrin was added to the reaction liquid, followed by heating to 100° C. and stirring for 1 hour. To this reaction liquid, 33.8 g of methacrylic acid was added, and the temperature was raised to 110° C. to make it react for 3 hours. Thereafter, it was dissolved in tetrahydrofuran (THF), and after obtaining a precipitate with water, it was dissolved in dich...
Synthetic example 3
[0082] After preparing a flask equipped with a mechanical stirrer, a thermometer, and a cooling jacket, 240 g of propylene glycol monomethyl ether and 100 g of bisphenol fluorene represented by Chemical Formula 1-1 were added under a nitrogen stream, and heated to 40° C. while stirring. After adding 0.288 g of dibutyltin dilaurate to the reaction liquid, the temperature of the reactor was raised to 65°C. While continuing to stir the reaction solution, 36.98 g of 4,4'-diphenylmethane diisocyanate was slowly added separately, and the absorption spectrum of the isocyanate group (2280 cm -1) disappeared, and 315.2 g of epichlorohydrin was added to the reaction liquid, followed by heating to 100° C. and stirring for 1 hour. To this reaction liquid, 33.8 g of methacrylic acid was added, and the temperature was raised to 110° C. to make it react for 3 hours. Thereafter, it was dissolved in tetrahydrofuran (THF), and after obtaining a precipitate with water, it was dissolved in dichl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com