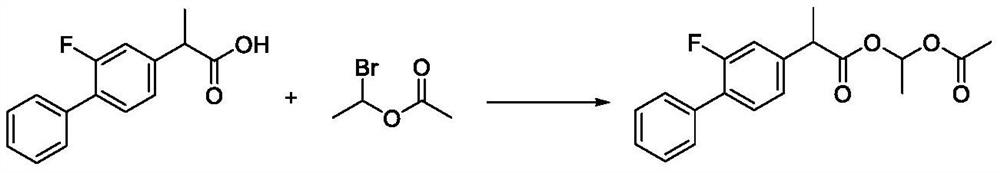
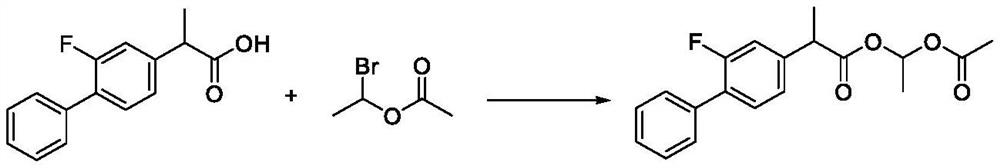
Preparation method of flurbiprofen axetil
A technology of flurbiprofen axetil and flurbiprofen, which is applied in the field of preparation of flurbiprofen axetil, can solve the problems of complex post-treatment operation, high reaction temperature, high purification cost, etc., achieve short reaction time, high purity, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Add 2.0kg of flurbiprofen and 15.6kg of acetone in a 50L reaction kettle equipped with a thermometer and a constant pressure dropping funnel, stir at room temperature (20-25°C), and dissolve the reaction solution, add 1.36kg of anhydrous potassium carbonate, and stir From 0.5 hour to 1 hour, 1.64 kg of 1-bromoethyl acetate was added dropwise, and the addition was completed in 5 to 15 minutes. Raise the temperature to an internal temperature of 55-60°C, and keep the reaction for 3 hours.
[0054] The reaction solution is concentrated under reduced pressure. When the vacuum degree reaches above 0.09Mpa and no liquid drops out, it is regarded as the end point of concentration. Add 18kg of ethyl acetate and 20kg of purified water to the reaction solution, stir for 30min, then let it stand for 15min, then remove the lower layer of water. Phase, the organic phase was washed with purified water (10kg × 3), added anhydrous magnesium sulfate 4kg and dried for 2 hours, filtered, ...
Embodiment 2
[0057] Add 2.0kg of flurbiprofen, 15.6kg of acetone and 2.35kg of N,N-dimethylformamide into a 50L reactor equipped with a thermometer and a constant pressure dropping funnel, at room temperature (20-25°C) Stir to dissolve the liquid, add 1.36 kg of anhydrous potassium carbonate, stir for 0.5 to 1 hour, add 1.64 kg of 1-bromoethyl acetate dropwise, and complete the dropwise addition in 5 to 15 minutes. React at 20-25°C for 4 hours.
[0058] The reaction solution is concentrated under reduced pressure. When the vacuum degree reaches above 0.09Mpa and no liquid drops out, it is regarded as the end point of concentration. Add 18kg of ethyl acetate and 20kg of purified water to the reaction solution, stir for 30min, then let it stand for 15min, then remove the lower layer of water. Phase, the organic phase was washed with purified water (10kg × 3), added anhydrous magnesium sulfate 4kg and dried for 12 hours, filtered, and concentrated to obtain 2.61kg of crude product flurbiprofe...
Embodiment 3
[0061] Add 2.0kg of flurbiprofen and 15.6kg of acetone and 3.76kg of N,N-dimethylformamide into a 50L reaction kettle equipped with a thermometer and a constant pressure dropping funnel, at room temperature (20-25°C) Stir to dissolve the liquid, add 1.36 kg of anhydrous sodium carbonate, stir for 0.5 to 1 hour, cool down to 10°C, add 2.05 kg of 1-bromoethyl acetate dropwise, and complete the dropwise addition in 5 to 15 minutes. Keep the reaction at 10-15°C for 8 hours.
[0062] The reaction solution is concentrated under reduced pressure. When the vacuum degree reaches above 0.09Mpa and no liquid drops out, it is regarded as the end point of concentration. Add 20kg of ethyl acetate and 20kg of purified water to the reaction solution, stir for 30min, then let it stand for 15min, and then remove the lower layer of water. Phase, the organic phase was washed with purified water (10kg × 3), added anhydrous sodium sulfate 4kg and dried for 12 hours, filtered, and concentrated to dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com