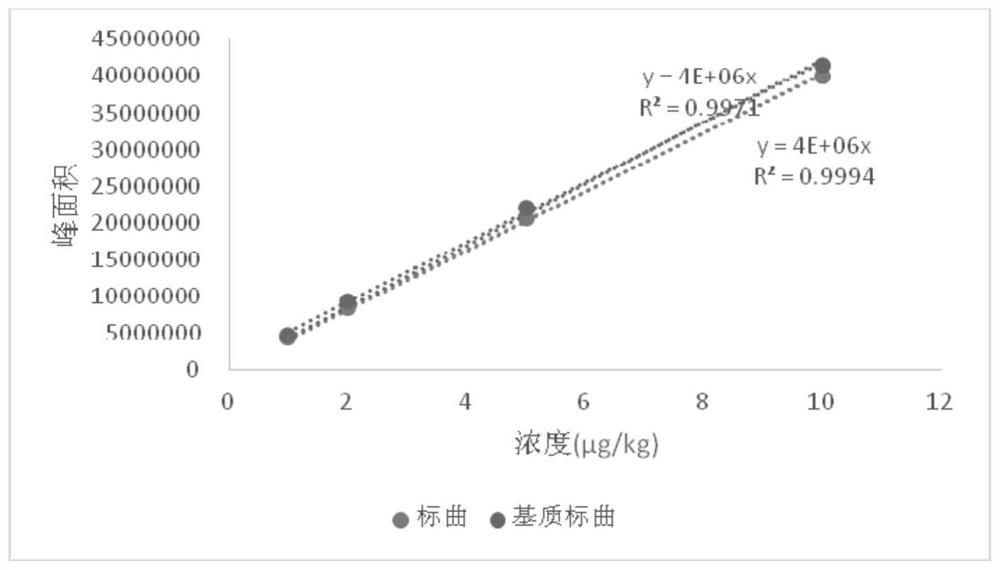
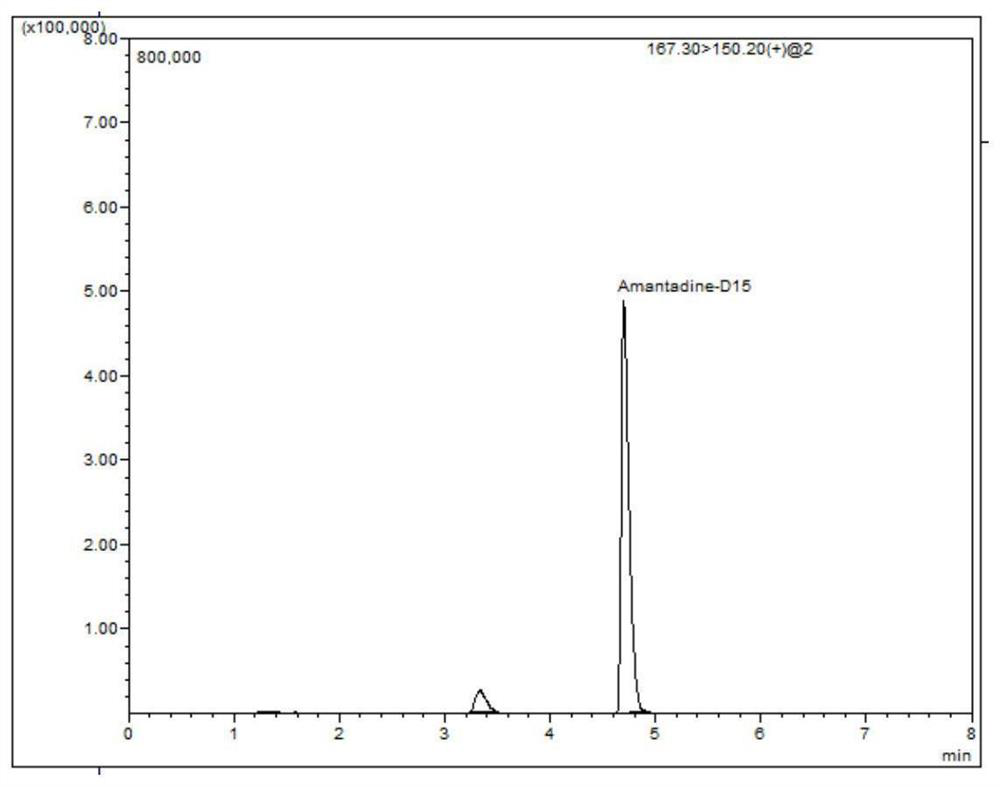
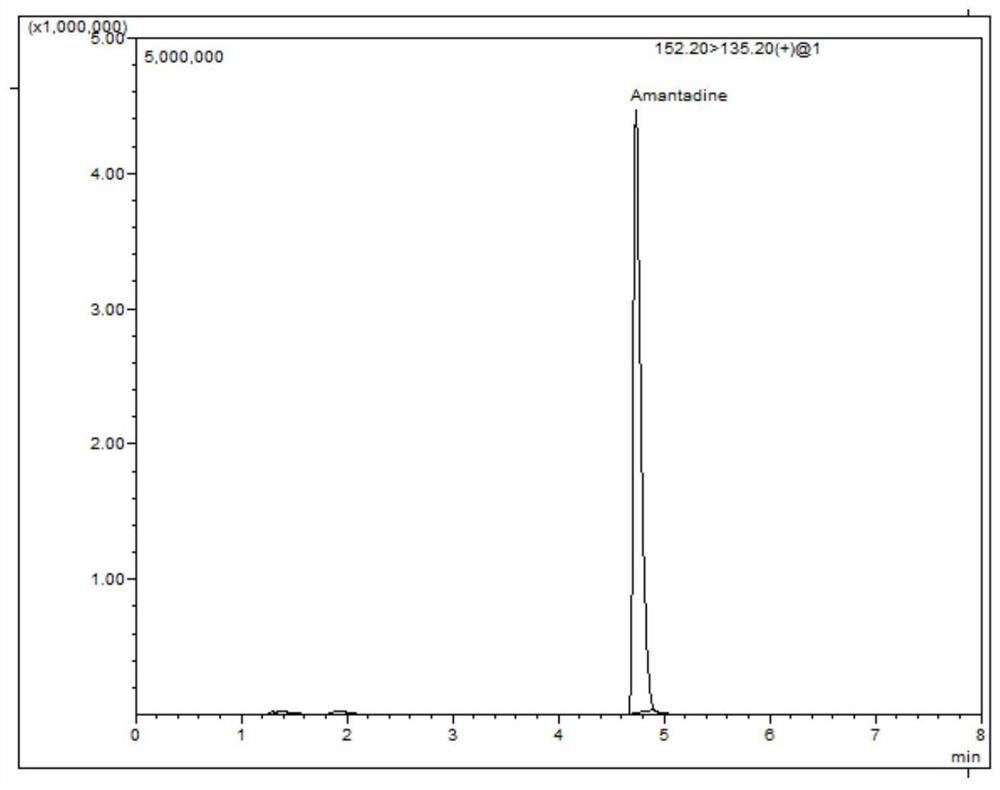
LC-MS/MS (Liquid Chromatography-Mass Spectrometry/Mass Spectrometry) determination method for residual quantity of amantadine in eggs
A LC-MS, amantadine technology, applied in the field of determination of veterinary drug residues in eggs, to achieve the effect of avoiding matrix interference, good repeatability, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Detection of amantadine residues in eggs
[0053] (1) extraction
[0054] Take the eggs to be tested, break them, stir the egg yolks and egg whites evenly with a homogenizer, weigh 2±0.02g samples into a 50mL centrifuge tube, add 100μL of 20μg / L internal standard, mix well, let stand for 30min, add 1% Acetic acid acetonitrile solution 10mL, vortex 2min, centrifuge at 10000r / min for 5min, transfer the supernatant to a 50mL centrifuge tube, repeat the extraction once, combine the supernatant twice, add n-hexane 15mL, vortex 1min, 10000r / min Centrifuge for 10 min, discard the n-hexane layer, and set aside.
[0055] (2) purification
[0056] Activate the mixed cation solid phase extraction column Oasis MCX with 3mL methanol, balance with 3mL water, then take the supernatant and put it on the column, the flow rate is controlled at 2-3 drops / s, rinse with 3mL 2% hydrochloric acid aqueous solution, 3mL methanol successively, pump Dry for 3min, elute with 5mL 5% a...
Embodiment 2
[0083] Example 2 Determination method
[0084] Qualitative determination: through the comparison of the retention time of the sample chromatogram with the retention time of the standard, the characteristic ions of the chromatographic peak and the characteristic ions of the chromatographic peak of the corresponding concentration standard. The relative deviation of the retention time between the sample and the standard is not more than 2.5%; the relative abundance of the characteristic ion of the sample is consistent with the relative abundance of the standard solution with equivalent concentration, and the relative abundance deviation does not exceed the provisions in Table 3, then it can be judged that the A corresponding analyte exists. The retention time deviation is within ±5%, and the relative abundance of the detected ions should be consistent with the relative abundance of the calibration standard solution with equivalent concentration. Its allowable deviation should me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com