COX-2 enzyme targeting phthalocyanine indometacin complex as well as preparation method and application thereof
A technology of COX-2 and indomethacin, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, can solve the problem of reducing the effect of photodynamic therapy, reducing the degree of phthalocyanine aggregation, Affect singlet oxygen and other issues to achieve the effect of increasing singlet oxygen production rate, reducing aggregation degree, and enhancing uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Y
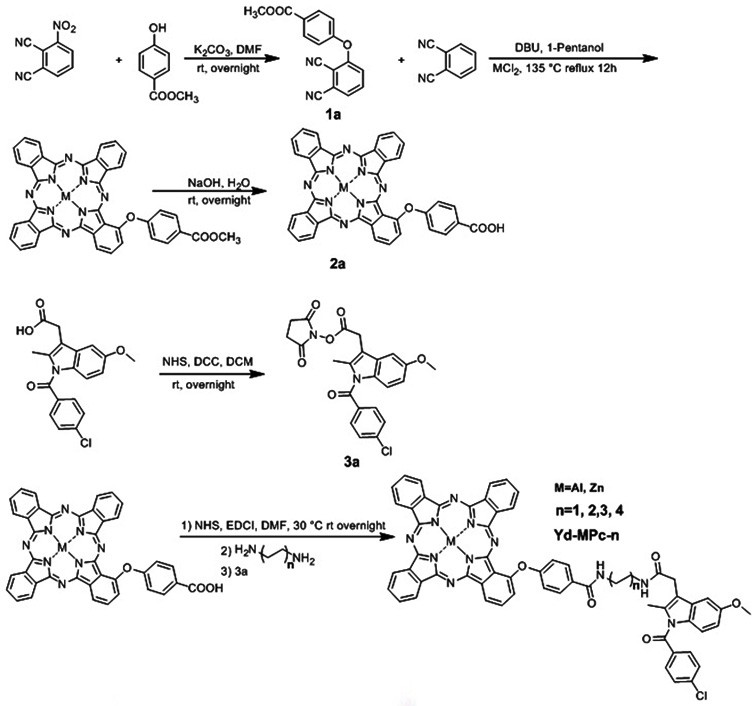
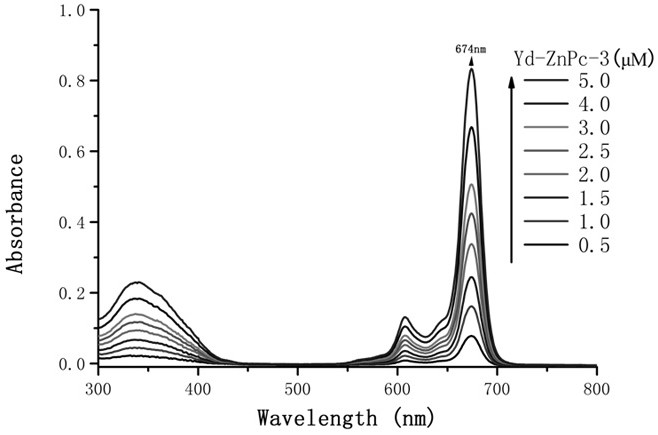
[0025] The preparation of embodiment Yd-ZnPc-3
[0026] With 3-nitrophthalonitrile as the original raw material, 3-(4-carboxymethylphenoxy)phthalonitrile is obtained through nucleophilic reaction, and then 1-(4- Carboxyphenoxy) zinc phthalocyanine, and then through the nucleophilic substitution reaction between 1-(4-carboxyphenoxy) zinc phthalocyanine, hexamethylenediamine, and indomethacin active ester to generate the target product of Yd-Pc.
[0027] Specific steps are as follows:
[0028] 1) Mix 2.00g (11.6mmol) 3-nitrophthalamide, 1.757g (11.6mmol) methyl p-hydroxybenzoate, 3.202g (23.2mmol) K 2 CO 3 Dissolved in 20 ml of DMF, reacted at room temperature for 24 hours, added 100 ml of water to the reaction solution to precipitate a white precipitate, filtered and dried to obtain 2.5 g of compound 1a with a yield of 78%. 1 H NMR (400 MHz, Chloroform- d ) δ 8.13 (d, J = 8.2 Hz, 2H), 7.64 (t, J = 8.2 Hz, 1H), 7.54 (d, J = 7.7 Hz, 1H), 7.16 (dd, J = 17.6, 8.2 Hz, 3H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com