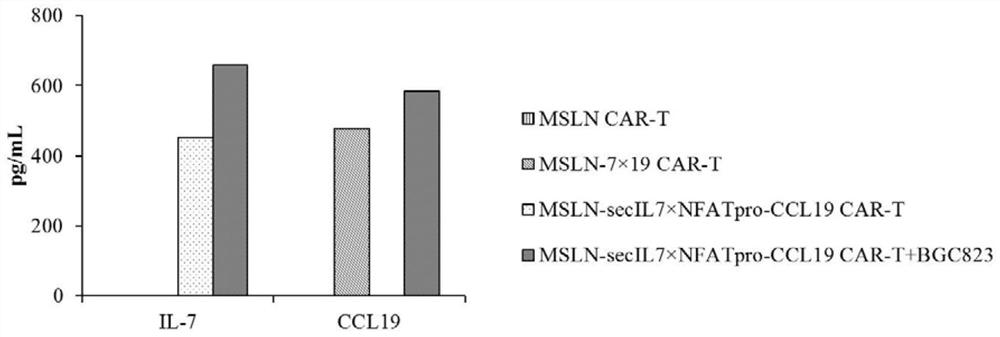
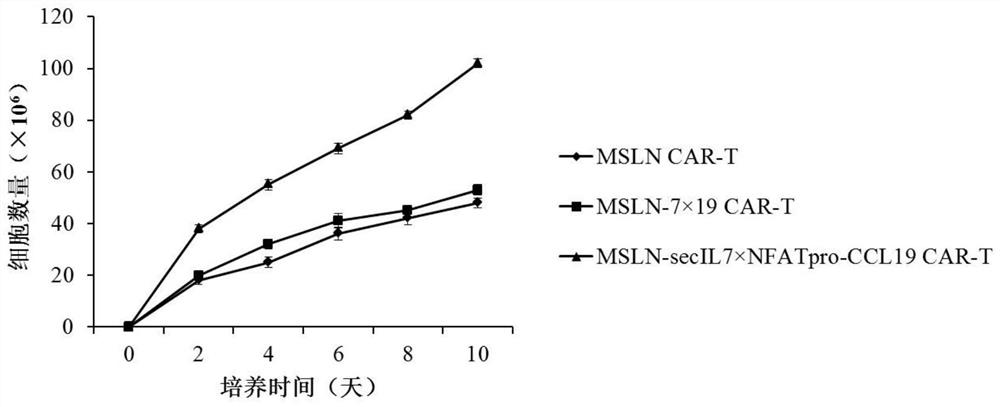
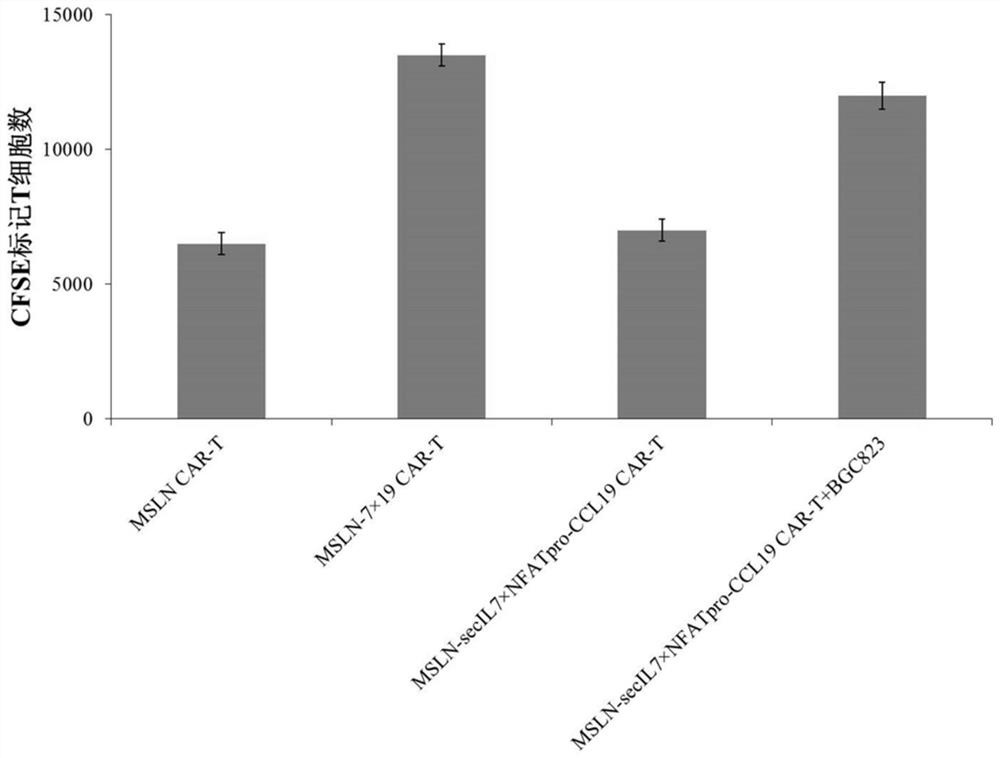
Expression vector for co-expressing secretory IL-7 and selective CCL19 and application of expression vector
An expression vector, IL-7 technology, applied in the field of biomedicine, can solve the problem of reducing the probability of T cells and dendritic cells being recruited into tumors, the inability to recruit peripheral blood immune cells, and the inability of exogenous IL-7 protein Secretion and other problems, to achieve the effect of increased proliferation ability and tumor cell killing efficiency, and low inflammatory side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056]Example 1 Preparation of Lentiviral Vector
[0057]The following nucleic acid molecules are synthesized by the whole gene:
[0058]① CAR molecule formed by the nucleic acid sequence of GM-CSF signal peptide, MSLN scFv, CD8α, 4-1BB and CD3ζ in series (amino acid sequence is shown in SEQ ID NO: 5);
[0059]② by IL-7 signal peptide (SEQ ID NO: 8) and IL-7 (without IL-7 signal peptide) (SEQ ID NO: 2), 2A peptide (SEQ ID NO: 9) and CCL19 (SEQ ID NO :4) nucleic acid molecules formed by tandem nucleic acid sequences;
[0060]③ A secreted IL-7 molecule formed by the nucleic acid sequence of IL-6 signal peptide (SEQ ID NO: 1) and IL-7 (without IL-7 signal peptide) (SEQ ID NO: 2) in series;
[0061]④ A selective CCL19 molecule formed by 2×NFAT-RE-minP promoter (SEQ ID NO: 3) and CCL19 (SEQ ID NO: 4) in series;
[0062]SEQ ID NO: 8:
[0063]atgttccatgtttcttttaggtatatctttggacttcctcccctgatccttgttctgttgccagtagcatcatct;
[0064]SEQ ID NO: 9:
[0065]aaaattgtcgctcctgtcaaacaaactcttaactttgatttactcaaactggctggggatgtagaaagc...
Embodiment 2
[0067]Example 2 Recombinant lentivirus packaging
[0068]In this example, 293T cells are used to prepare recombinant lentivirus. When the 293T cells are spread on a 100mm culture dish to 80-90% of the bottom, lentivirus packaging is performed:
[0069]2h before virus packaging, change the medium to DMEM containing 1% fetal bovine serum, and add 6mL / 100mm petri dish;
[0070]Prepare the plasmid mixture as shown in Table 1. The pWPXLd-expression plasmid includes a lentiviral vector expressing CAR molecule, a lentiviral vector expressing intact IL-7 and CCL19, or a lentiviral expressing secreted IL-7 and selective CCL19 Carrier
[0071]Table 1
[0072]
[0073]
[0074]Add 36 μg PEI to another 500 μL opti-MEM medium, mix well, and let stand at room temperature for 5 minutes;
[0075]Mix the plasmid mixture shown in Table 1 with PEI, mix by pipetting, and let stand at room temperature for 25-30 minutes;
[0076]Add the above mixture dropwise to the 293T cells cultured in a 100mm petri dish;
[0077]After culturing f...
Embodiment 3
[0080]Example 3 T cell activation and lentiviral transfection
[0081]Use Ficoll density gradient centrifugation kit (GE company) to separate peripheral blood mononuclear cells (PBMC) from whole blood, remove red blood cells, and then use MACS Pan-T magnetic beads to sort out T cells;
[0082]The sorted T cells are diluted with medium (AIM-V medium + 5% FBS + penicillin 100 U / mL + streptomycin 0.1 mg / mL) to a cell concentration of 2.5×106Pieces / mL to be used;
[0083]Use CD2 / CD3 / CD28 T cell activation amplification kit (Miltenyi) to activate T cells, that is, the coated magnetic beads are mixed with T cells in a ratio of 1:2, and the final density of T cells is 5×106Piece / mL / cm2, After mixing, place at 37℃, 5% CO2Incubate for 48h;
[0084]After T cells are activated for 48 hours, demagnetize the beads, centrifuge at 300g for 5 min, remove the supernatant, resuspend the T cells in fresh medium, add recombinant lentivirus expressing CAR, recombinant lentivirus expressing intact IL-7 and CCL19 or ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com