Sulfide solid electrolyte sheet and preparation method thereof
A technology of sulfide electrolyte and solid electrolyte, which is used in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, circuits, etc., can solve the problem of low ionic conductivity and so on.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
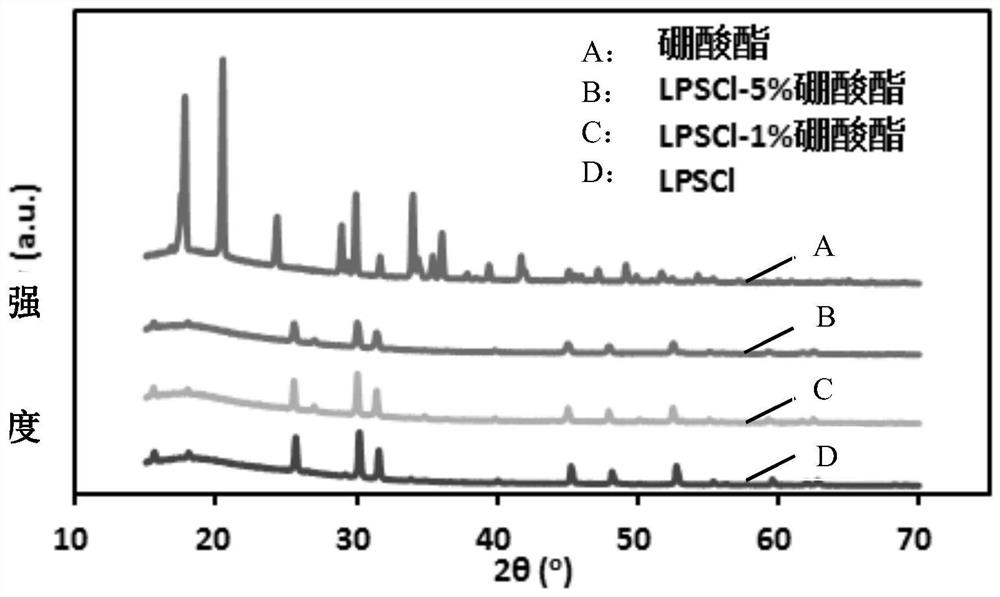
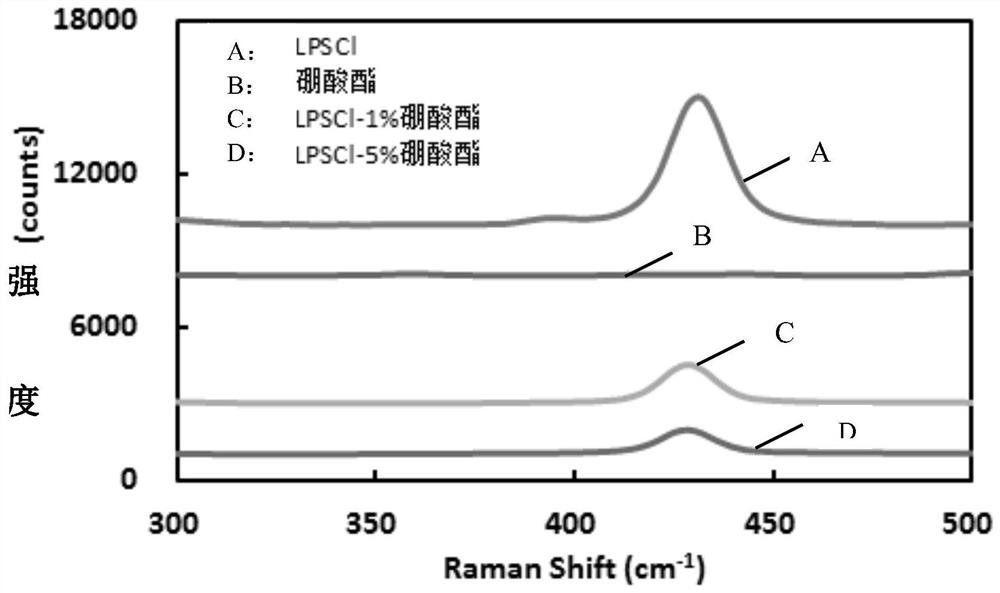
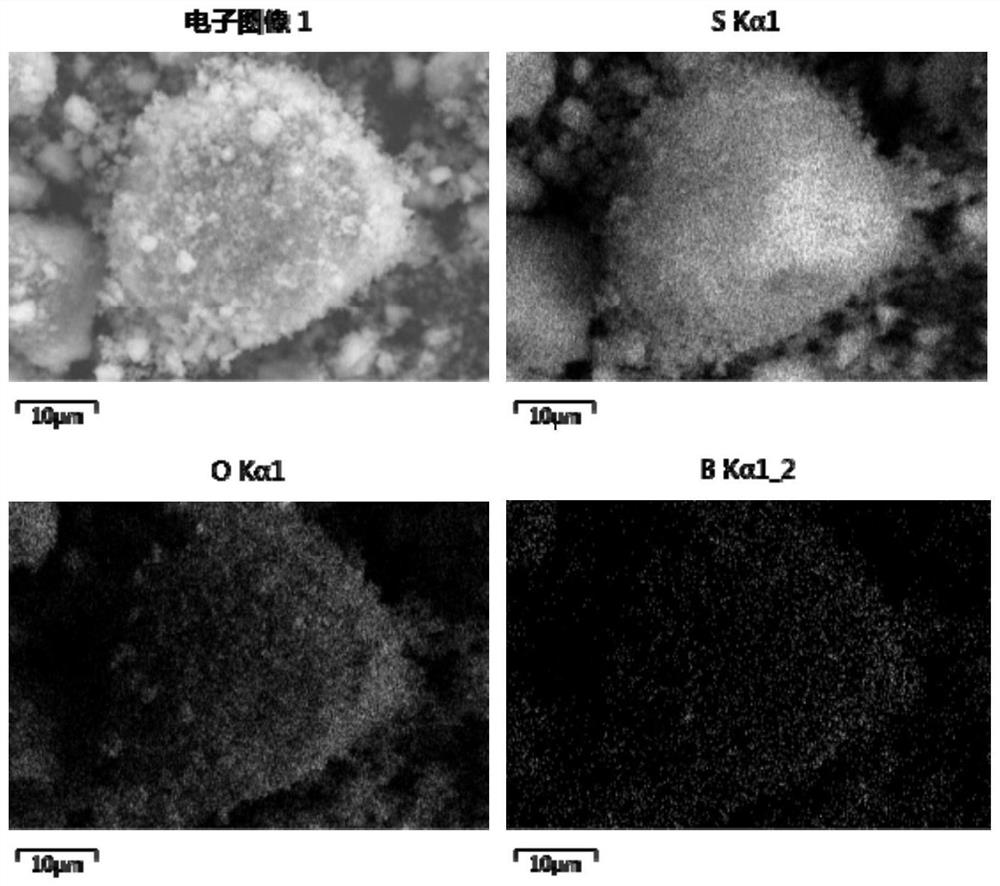
[0028]In the second aspect of the present invention, the embodiment of the present application also provides the preparation method of the above-mentioned sulfide solid electrolyte sheet, including: dispersing the sulfide electrolyte raw material in an organic solvent to form a reaction mixture; dispersing the borate ester in the organic solvent In the process, a modified solution is formed; the initial reaction mixture is mixed with the modified solution, and dried to obtain an initial product; the initial product is post-treated more than once, and each post-treatment includes grinding, cooling, pressing and sintering steps.
[0029] In the preparation method provided in the embodiments of the present application, borate is used as the doping raw material to obtain a solid electrolyte co-doped with boron and oxygen, wherein the doping of boron element can reduce the binding effect of anion on lithium ions and increase the concentration of lithium ions. The transmission abili...
Embodiment 1
[0082] The data of Examples 1-5 show the influence of the content change of boron element in the electrolyte sheet on the performance of the electrolyte sheet. When the content of boron element in the electrolyte sheet is too low (as in Example 1), the introduction of boron element has little effect on the electrolyte structure, although the conductivity of the electrolyte sheet can also be improved to a certain extent, but the effect of this improvement is not significant , and too low boron element content will lead to increased concentration test error, so the measured concentration deviation is also large. When the content of boron element in the electrolyte sheet is too high, the problem of local element enrichment is prone to occur, which is not conducive to achieving uniform modification results, and too high boron doping amount will reduce the relative concentration of lithium ions in the electrolyte sheet. Therefore, it is not conducive to improving the conductivity a...
Embodiment 3
[0083] The data of Examples 3 and 6 show the influence of the number of post-treatments on the performance of the electrolyte sheet. It can be seen from Table 1 that the performance of Example 6 is better than that of Example 3. This is because, as the number of post-treatments increases, the doped electrolyte material undergoes multiple grinding, briquetting and sintering. The distribution of various elements in the bulk material tends to be uniform, and the surface roughness and interface resistance are reduced. However, too many times of high-temperature sintering may also cause structural phase changes in the electrolyte, which will affect the increase in conductivity. Therefore, in the embodiment of the present application, the number of post-treatments should be more than 1 time, preferably 2-3 times.
[0084] Examples 6-12 take three post-treatments as an example to illustrate the technical effects produced by the gradient increase of the sintering temperature as the n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface roughness | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com