Dihydromyricetin gastric floating pills and preparation method thereof
A technology of dihydromyricetin and floating pills, applied in the field of medicine, can solve the problems of poor solubility, easy drug leakage, drug stability, low encapsulation rate, etc., and achieves the effects of expanding output, improving floating rate and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
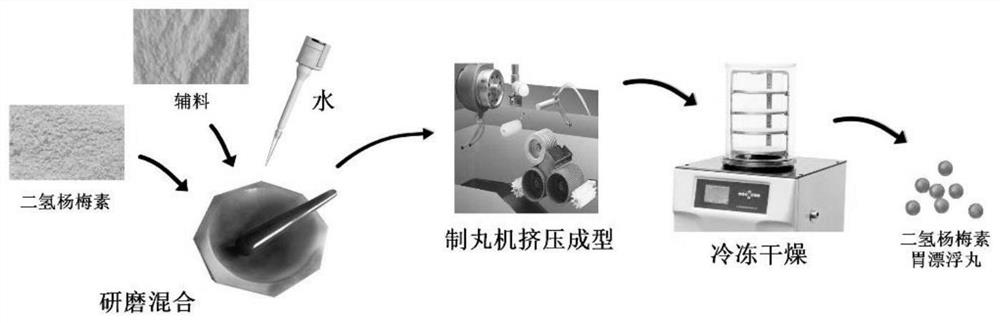
[0048] The preparation method of embodiment 1-10 is as follows:
[0049] Step 1: Add dihydromyricetin, 1-hexadecanol, hyaluronic acid, PVP-K30 and sodium bicarbonate into a marble mortar.
[0050] Step 2: Add purified water and grind thoroughly to form a paste mixture.
[0051] Step 3: Transfer the pasty mixture to the HK-93C high-performance pellet machine to make spherical pre-pellets with a diameter of about 3-8 mm.
[0052] Step 4: Freeze-dry the spherical pre-pellet for 12-36 hours to obtain the final product.
Embodiment 1
[0054] (1) Take 200 mg of dihydromyricetin, 90 mg of 1-hexadecanol, 130 mg of hyaluronic acid, 70 mg of PVP-K30, and 50 mg of sodium bicarbonate into a marble mortar.
[0055] (2) Add 225 μl of purified water, grind thoroughly to form a paste mixture.
[0056] (3) Transfer the pasty mixture to the HK-93C high-performance pellet machine to make spherical pre-pellets with a diameter of about 3-8 mm.
[0057] (4) The spherical pre-pellet is freeze-dried for 12-36 hours to obtain the final product.
Embodiment 2
[0059] (1) Take 200 mg of dihydromyricetin, 90 mg of 1-hexadecanol, 140 mg of hyaluronic acid, 80 mg of PVP-K30, and 50 mg of sodium bicarbonate into a marble mortar.
[0060] (2) Add 263 μl of purified water, grind thoroughly to form a paste mixture.
[0061] (3) Transfer the pasty mixture to the HK-93C high-performance pellet machine to make spherical pre-pellets with a diameter of about 3-8 mm.
[0062] (4) Freeze-drying the spherical pre-pellet for 12-36 hours to obtain the final product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com