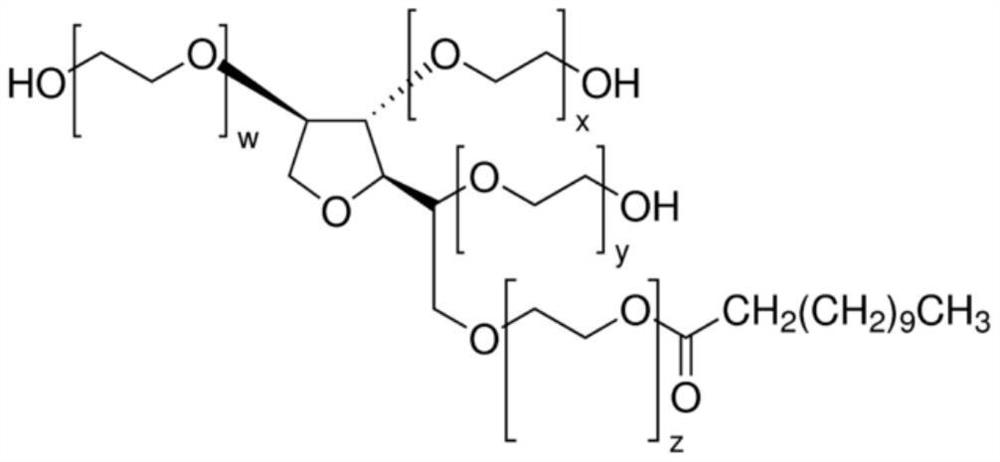
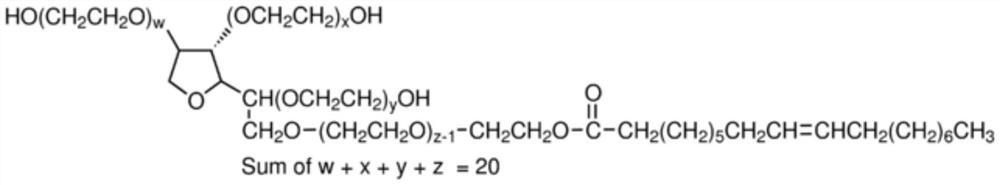
Germ-repellent plastic, a gas pathway of a respiratory device containing, and a manufacturing method therefor
A technology for antibacterial plastics and breathing devices, applied in the field of plastics, can solve the problems of technical limitations, reducing the design flexibility of medical breathing device manufacturers, and increasing costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063]Preparation of bacterial test inoculum: Escherichia coli ( 8739 TM ) and Staphylococcus aureus ( 6538P TM ).
[0064] According to Japanese Industrial Standards (JIS Z 2801:2000), a test inoculum of bacteria was prepared and counted while culturing. With slight modifications, this JIS was used to test the antimicrobial activity and efficacy against bacteria on the surface of the sample. Please refer to Flowchart 1 to illustrate the process in graphical format with the following explanation:
[0065]
[0066] Flowchart 1 Preparation of test inoculum of bacteria
[0067] 1) Pick out a single bacterial colony from the agar plate (stored in a refrigerator at 4°C), and transfer it to 3 mL of gravy for overnight culture;
[0068] 2) Transfer 300 μL of cultured bacteria to 3 mL of fresh gravy and incubate for about 3-4 hours;
[0069] 3) Harvest bacteria by centrifuging at a speed of 8000rpm for 1-2 minutes (for Escherichia coli, OD600 must be adjusted to 0.6-0.7; f...
Embodiment 1
[0095] A mixture of 8.3 wt% EVA base plastic, 83.3 wt% ethylene / acrylic acid / maleic acid terpolymer intermediate plastic, and 8.3 wt% ceteareth anti-biofouling compound was mixed to form a masterbatch. Specifically, the anti-biofouling compound, EVA base plastic and intermediate plastic were mixed together in a twin-screw extruder whose temperature ranged from 90°C at the front to 190°C at the rear.
[0096] The (main) base plastic is a blend of two ethylene-octene POEs. The weight ratio of masterbatch to base plastic is 1:54. The masterbatch and base plastic were fed into a single-screw extruder whose temperature ranged from 90°C at the front to 235°C at the rear to form the antimicrobial plastic (A). The single-screw extruder extrudes antimicrobial plastic in sheet form.
[0097] Therefore, the percentage by weight in the final antibacterial plastic sheet is: 0.15% EVA base plastic, 1.5% ethylene / acrylic acid / maleic acid terpolymer intermediate plastic, 0.15% ceteareth ant...
Embodiment 2
[0108] Antibacterial plastics (B) and comparative plastics (comparison 2) were prepared according to the method of Example 1, except that the temperature range of the twin-screw extruder was 110° C. at the front to 190° C. at the rear when preparing the masterbatch. When preparing antibacterial plastics, the temperature of the single-screw extruder ranges from 210°C at the front to 235°C at the rear. In addition, these samples were extruded from a single screw extruder to form lengths of tubing rather than sheets as in Example 1.
[0109] A swab test (above) was performed comparing the antimicrobial properties of the antimicrobial plastic (B) and a control plastic (Control 2).
[0110] Example 2 Table 1
[0111] Tube samples with E. coli copy 1 copy 2 copy 3 average Comparative Plastic (Comparison 2) 2.44x 10 3
1.01x 10 4
6.22x 10 3
6.25x 10 3
Antibacterial plastic (B) 3.60x 10 2
3.20x 10 2
1.43x 10 2
2.74x 10 2
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melt flow index | aaaaa | aaaaa |
| melt flow index | aaaaa | aaaaa |
| melt flow index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com