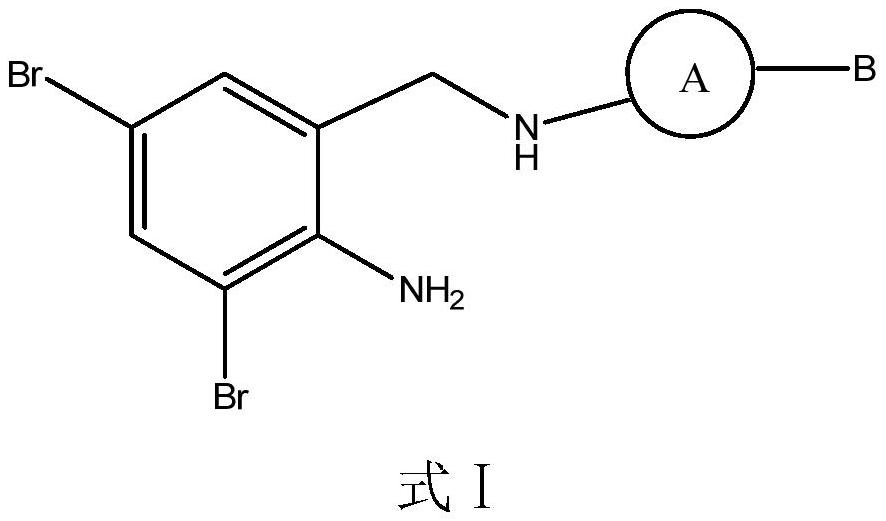
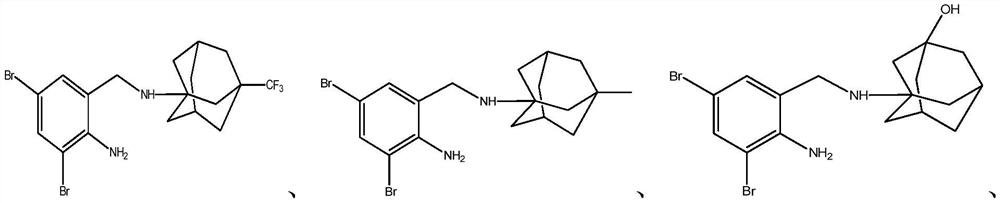
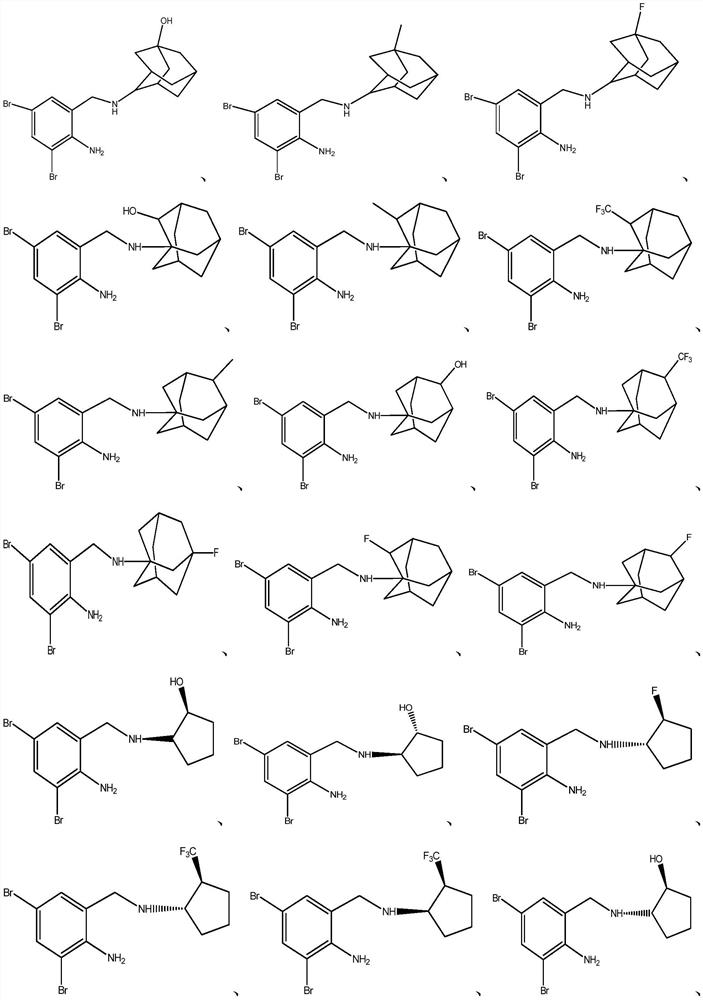
Dibromobenzyl derivative, stereoisomer or salt thereof, and preparation method and application thereof
A stereoisomer, dibromobenzyl technology, applied in the field of its stereoisomer, dibromobenzyl derivatives, can solve problems such as unsatisfactory expectorant effect and reduced metabolic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] This example discloses the preparation of compound 1: 3-((2-amino-3,5-dibromobenzyl)amino)adamantan-1-ol, whose structure is as follows:
[0046]
[0047] Add 3 g (0.018 mol) of 3-amino-1-adamantanol, 10 g (0.036 mol) of 2-amino-3,5-dibromobenzaldehyde and 100 ml of tetrahydrofuran (THF) into the reaction flask, reflux overnight, and monitor the reaction with TCL , until the raw material drug basically disappeared, cooled to room temperature, added 2.5g (0.066mol) of sodium borohydride to react for 24 hours, then added about 250ml of water, then extracted with 300ml of ethyl acetate, concentrated under reduced pressure to obtain compound 1, a total of 6.60g, HPLC The purity is 98.70%, and the yield is 86%.
[0048] MS m / z(ES):429.01
[0049] 1H NMR (400MHz,D 2 O)δ7.81(d,1H),7.21(d,1H),3.76(s,2H),1.76(s,2H),1.68-1.66(m,6H),1.46-1.55(m,4H), 1.34-1.38 (m, 4H).
Embodiment 2
[0051] This example discloses the preparation of compound 2: 3-((2-amino-3,5-dibromobenzyl)amino)adamantan-1-ol hydrochloride, whose structure is as follows:
[0052]
[0053] Compound 1 was prepared according to the method of Example 1, and then (0.018mol) Compound 1 was washed with 100ml of saturated sodium chloride, acidified with hydrochloric acid, and concentrated to dryness under reduced pressure to obtain Compound 2, a total of 7.06g, HPLC purity 98.65%, yield 85%.
[0054] MS m / z(ES):429.02
[0055] 1H NMR (400MHz,D 2O)δ7.80(d,1H),7.22(d,1H),3.76(s,2H),1.77(s,2H),1.68-1.67(m,6H),1.46-1.56(m,4H), 1.34-1.38 (m, 4H).
Embodiment 3
[0057] This example discloses the preparation of compound 3: (1R,2R)-2-((2-amino-3,5-dibromobenzyl)amino)-cyclopentanol, whose structure is as follows:
[0058]
[0059] Add 3 g (0.029 mol) of trans (1R, 2R)-2-aminocyclopentanol, 10 g (0.036 mol) of 2-amino-3,5-dibromobenzaldehyde and 100 ml of THF into the reaction flask, reflux overnight, and TCL Monitor the reaction until the raw material drug basically disappears, cool to room temperature, add 2.5g (0.066mol) of sodium borohydride to react for 24 hours, then add about 250ml of water, then extract with 300ml of ethyl acetate, and concentrate under reduced pressure to obtain compound 3, a total of 8.90g , HPLC purity 98.76%, yield 86%.
[0060] MS m / z(ES):362.95
[0061] 1H NMR (400MHz,D 2 O)δ7.81(d,1H),7.28(d,1H),3.45(s,2H),4.22(m,1H),3.59(m,1H),2.09-2.34(m,2H),1.53- 1.72 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com