Method for preparing and separating 5-hydroxymethylfurfural from fructose
A technology of hydroxymethylfurfural and fructose, which is applied in the field of biomass catalytic conversion, can solve the problems of large consumption of reaction solvent, low initial sugar concentration, and uneconomical problems, and achieve the goal of reducing the amount, improving economy, and increasing yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
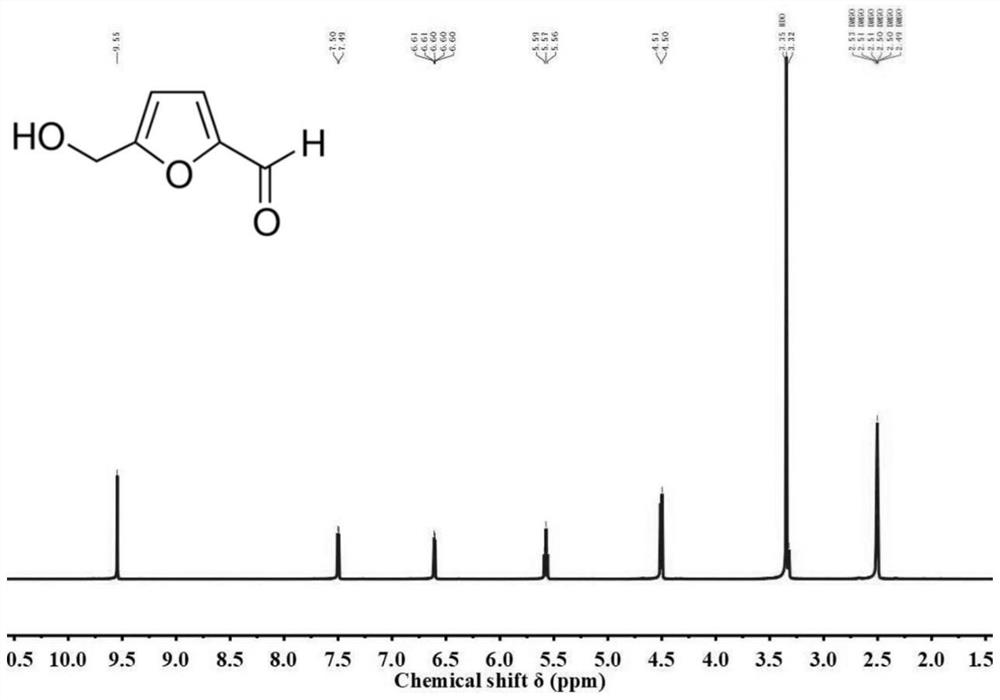
[0026] Weigh 3.0 g of fructose and 1.5 g of choline chloride and mix them in a 50 mL round bottom flask, heat at 20° C. until it completely melts to a colorless transparent liquid to obtain a fructose-choline chloride mixed solution. Then add 0.15 g of phosphotungstic acid and 30 mL of methyl isobutyl ketone, stir at 120° C., condense and reflux, and react for 1 h. Then collect the upper layer in the round-bottomed flask, carry out vacuum distillation through a rotary evaporator, recover the evaporated organic solvent, and use it as a solvent for the next experiment, and dry and cool the remaining product to obtain the product 5-hydroxymethyl Base furfural quality is 2.56g, utilizes liquid nuclear magnetic resonance analysis to know that its purity is 92%.
Embodiment 2
[0028] Weigh 3.0 g of fructose and 1.5 g of choline chloride and mix them in a 50 mL round bottom flask, heat at 30° C. until it completely melts to a colorless transparent liquid to obtain a fructose-choline chloride mixed solution. Then add 0.15g of phosphotungstic acid and 30mL of methyl isobutyl ketone, stir at 120°C, condense and reflux, and react for 1.5h. Then collect the upper layer in the round-bottomed flask, carry out vacuum distillation through a rotary evaporator, recover the evaporated organic solvent, and use it as a solvent for the next experiment, and dry and cool the remaining product to obtain the product 5-hydroxymethyl Base furfural quality is 2.60g, utilizes liquid nuclear magnetic resonance analysis to know that its purity is 93%.
Embodiment 3
[0030] Weigh 0.5g of fructose and 3.0g of choline chloride and mix them in a 50mL round bottom flask, heat at 80°C until it completely melts to a colorless transparent liquid to obtain a fructose-choline chloride mixed solution. Then add 0.025g of phosphotungstic acid and 2.5mL of methyl isobutyl ketone, stir at 80°C, condense and reflux, and react for 1.5h. Then collect the upper layer in the round-bottomed flask, carry out vacuum distillation through a rotary evaporator, recover the evaporated organic solvent, and use it as a solvent for the next experiment, and dry and cool the remaining product to obtain the product 5-hydroxymethyl Base furfural quality is 0.33g, utilizes liquid nuclear magnetic resonance analysis to know that its purity is 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com