Preparation method of vonoprazan intermediate
A compound and reaction technology, applied in the field of preparation of vonolazan intermediates, can solve the problems of harsh reaction conditions and cumbersome operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] The preparation method of Weissmeier reagent:
[0066] Add N,N-dimethylformamide into dichloromethane, cool down to 0°C, add phosphorus oxychloride dropwise, stir for 30
[0067] The solution obtained in minutes.
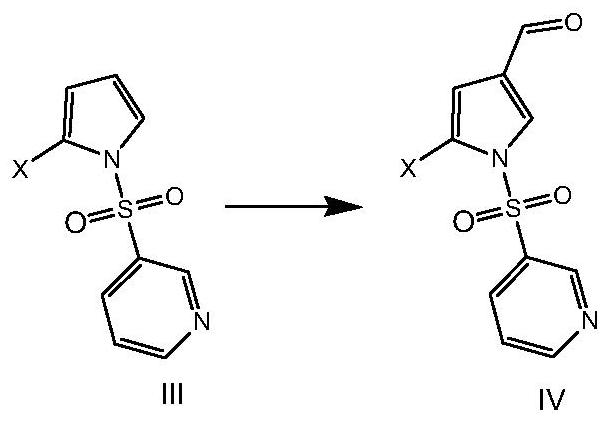
[0068] The preparation method of formula V compound
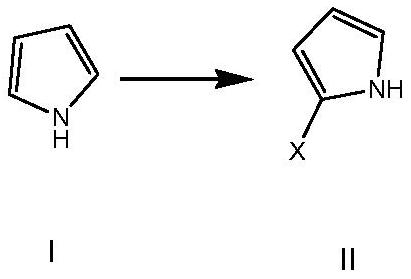
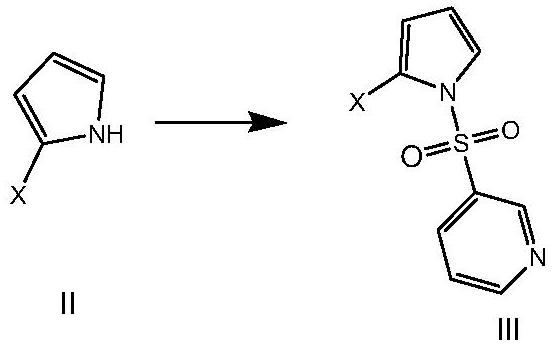
[0069] Aiming at the defects of the existing preparation methods of the compound of formula V, the present invention provides a novel preparation method of the compound of formula V, which uses pyrrole as a raw material and prepares the compound of formula V, an intermediate of vonorazan, under mild reaction conditions. Described method specifically comprises the steps:
[0070]
[0071] (1) In the first solvent, in the presence of a brominating reagent, the compound of formula I undergoes a bromination reaction to obtain the compound of formula II;
[0072] (2) In the second solvent, in the presence of a base, a sulfonylation reaction occurs between the compound of formula II and pyridine-3-sulfonyl...
Embodiment 1
[0105] Synthesis of embodiment 1 2-bromopyrrole
[0106]
[0107] Add tetrahydrofuran (100mL) to pyrrole (20g), cool down to -15°C, add N-bromosuccinimide (63.4g) in batches, keep warm until the reaction is complete, add ice-water solution, and extract the aqueous phase with ethyl acetate , the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain the target compound (39.4 g), yield: 91%. MS (ESI): [M+1] + = 145.94.
Embodiment 2
[0108] Example 2 Synthesis of 3-(2-bromo-pyrrole-1-sulfonyl)-pyridine
[0109]
[0110] Add 2-bromopyrrole (20g) into acetonitrile (100mL), add triethylamine (20.9g), add pyridine-3-sulfonyl chloride (29.3g) dropwise at room temperature, after the addition is complete, react at 40°C until the reaction is complete, add dilute Adjust the pH to 4-5 with hydrochloric acid, add water (200 mL) dropwise, add ethyl acetate for extraction, wash the organic phase with saturated brine, and concentrate to dryness to obtain 35.5 g of the target compound, yield: 90%. MS (ESI): [M+1] + = 286.95.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com