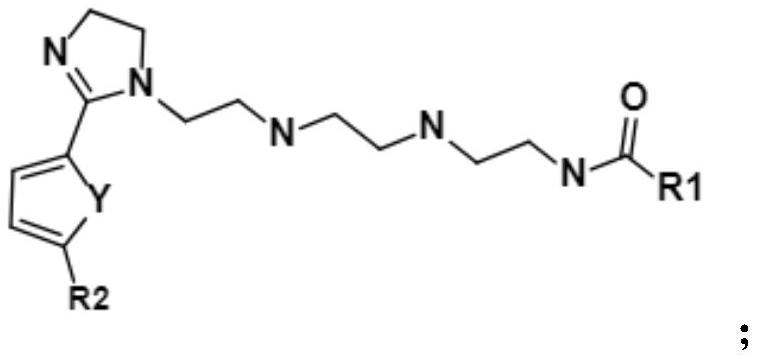
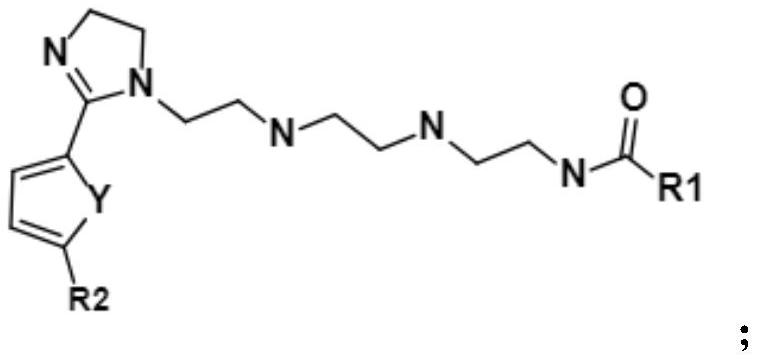
Imidazoline corrosion inhibitor with asymmetric end groups, preparation method and application thereof
An imidazoline corrosion inhibitor and imidazoline-based technology, applied in the direction of organic chemistry, can solve the problems of lack of corrosion inhibition and high imidazoline corrosion inhibitor, and achieve corrosion prevention, simple synthesis process and good corrosion inhibition effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
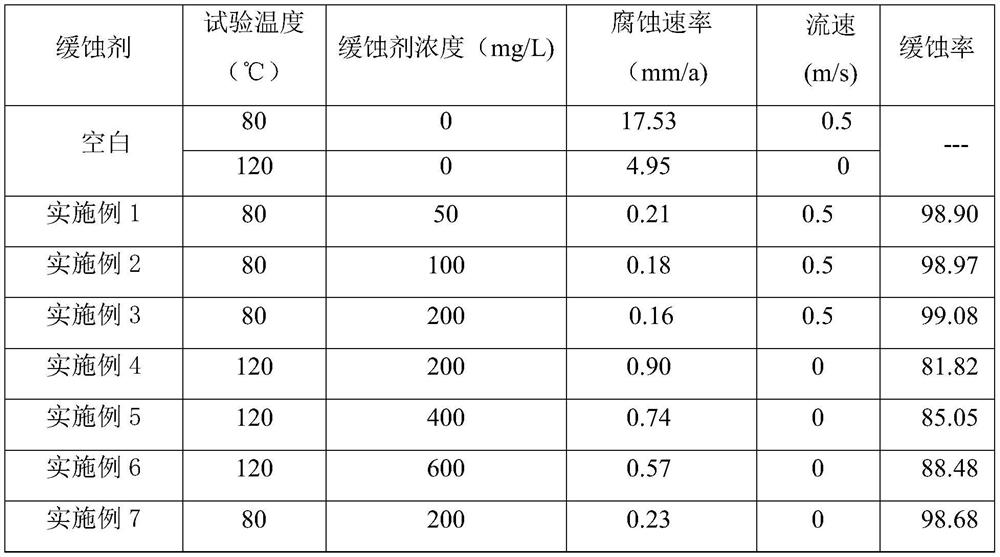
Embodiment 1
[0026] (1) Add solid 2-furoic acid and tetraethylenepentamine in a three-necked flask, add xylene water-carrying agent and the water generated by the reaction system to form an azeotrope, and carry the water out of the reaction, add zeolite, at 120 Amidation at ℃ for 3 hours, and then further heating to 220 ℃ for dehydration and cyclization for 2 hours, the reaction ratio of 2-furancarboxylic acid and tetraethylenepentamine is 1:1.05;
[0027] (2) After cooling the reaction system to 120° C., an equal proportion of oleic acid was added to continue the reaction for two hours. After the reaction was completed, water and xylene were removed by rotary evaporation.
Embodiment 2
[0029] (1) Add solid 2-thiophenecarboxylic acid and tetraethylenepentamine in a three-necked flask, add xylene water-carrying agent and the water generated by the reaction system to form an azeotrope, and carry the water to distill out the reaction, add zeolite, at 120 Amidation at ℃ for 3 hours, and then further heating to 220 ℃ for dehydration and cyclization for 2 hours, the reaction ratio of 2-furancarboxylic acid and tetraethylenepentamine is 1:1.05;
[0030] (2) After cooling the reaction system to 120° C., an equal amount of oleic acid was added to react for two hours. After the reaction was completed, the water and xylene in the system were removed by rotary evaporation.
Embodiment 3
[0032] (1) Add 2-pyrrole carboxylic acid and tetraethylenepentamine into a three-necked flask, add xylene water-carrying agent and water generated by the reaction system to form an azeotrope, and carry the water out of the reaction, add zeolite, and heat at 120°C Amidation under low temperature for 3 hours, and then further heating to 220°C for dehydration and cyclization for 2 hours, the reaction ratio of the 2-pyrrole carboxylic acid and tetraethylenepentamine is 1:1.05;
[0033] (2) After cooling the reaction system to 120° C., an equal amount of oleic acid was added to continue the reaction for 2 hours. After the reaction was completed, water and xylene were removed by cooling and rotary evaporation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com