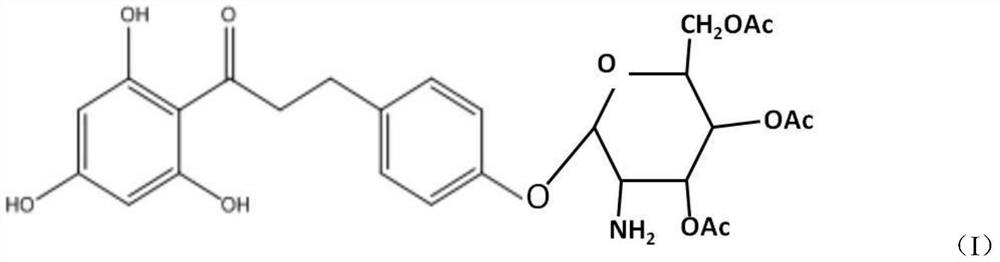
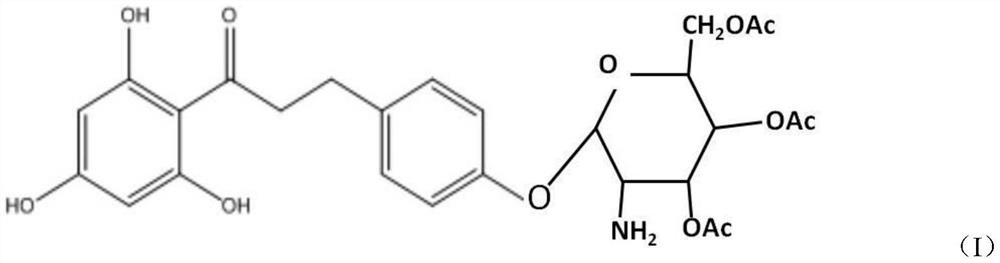
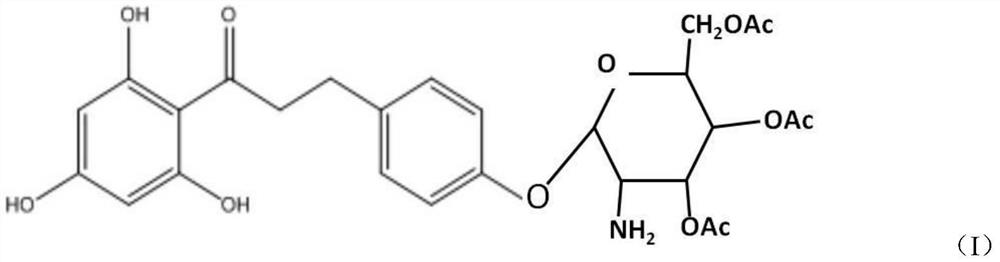
Cosmetic comprising phloretin derivative containing phloroglucinol groups and preparation method of phloretin derivative
A technology for phloretin and derivatives is applied in the field of cosmetics and preparations of phloretin derivatives, which can solve the problems of low skin absorption efficiency, poor water solubility of phloretin, limited application diversity of phloretin, and the like, and achieve excellent Antioxidative properties, improvement of water solubility and skin absorption efficiency, effect of securing antioxidative properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] (1), preparation of bromotetraacetylglucose
[0028] Add glucosamine to 3-5 times the molar amount of acetic anhydride, add 5-6 times the molar amount of triethylamine dropwise under ice-water bath conditions, add sufficient water to precipitate the product after reacting for 2 hours, filter and wash the filter cake with water After vacuum drying, aminotetraacetylglucose was obtained; the dried aminotetraacetylglucose was dissolved in dichloromethane, and hydrobromic acid of 2.5-4 times the molar amount of aminotetraacetylglucose was added dropwise at room temperature, wherein the hydrobromic acid was measured by mass Provide 30% hydrobromic acid acetic acid solution, react for 1.5-3 hours, pour into ice water, extract the water phase with dichloromethane, combine the organic phase, wash the organic phase quickly with saturated sodium bicarbonate solution and saturated saline respectively The pH value is 7, dried over anhydrous magnesium sulfate and filtered, and the fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com