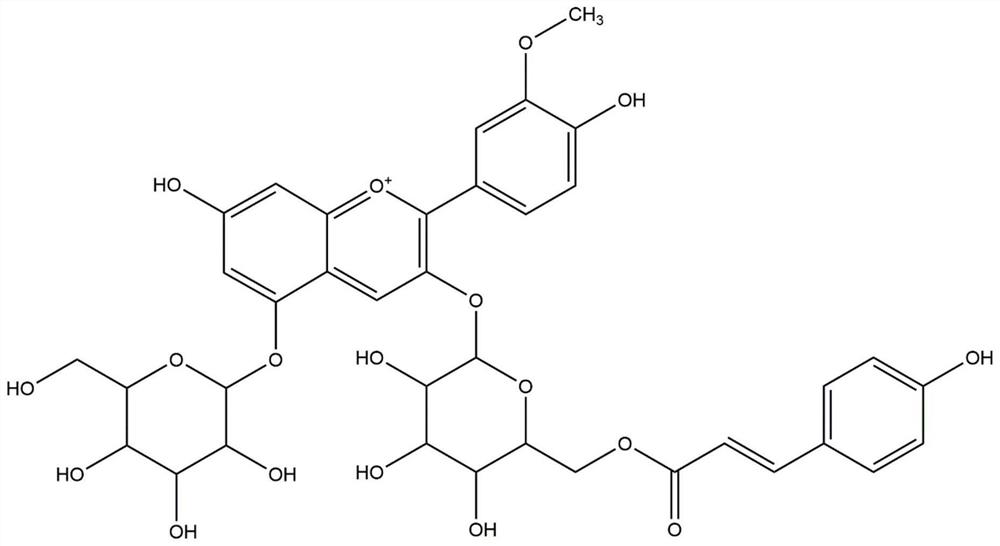
Separation preparation method of peonidin-3-O-(6-O-p-coumaroyl) glucoside-5-glucoside
A technology of glucoside and coumaroyl, which is applied in the field of separation and purification of natural products, can solve the problems of no acylated anthocyanins, difficulty in separation and purification of acylated anthocyanins, and difficulty in obtaining target anthocyanins, and achieves good repeatability and processing efficiency. large amount of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
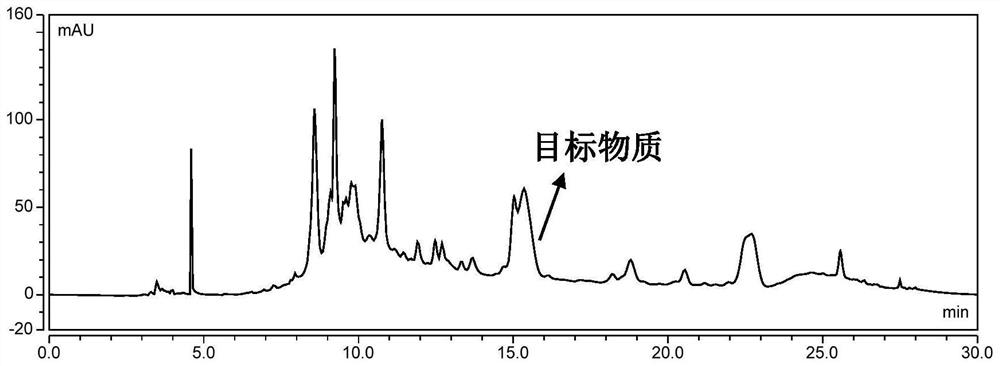
[0047] Wash and peel the grapes to obtain 1 kg of grape skins, add 70% ethanol solution containing 0.5% (v / v) hydrochloric acid according to the ratio of solid to liquid 1g: 8mL, mix well, and ultrasonically extract for 60 minutes, (control the temperature below 50°C , protected from light), filtered through gauze, the filtrate was centrifuged at 4000rpm for 10min, and the supernatant was taken. The filter residue was extracted once more in the same way. The filtrates were combined and filtered one more time using a Buchner funnel. The filtrate was evaporated under reduced pressure at 40°C to remove ethanol and concentrated to obtain a crude grape skin anthocyanin extract. The high performance liquid chromatogram of grape skin anthocyanin crude extract is as follows figure 2 shown.
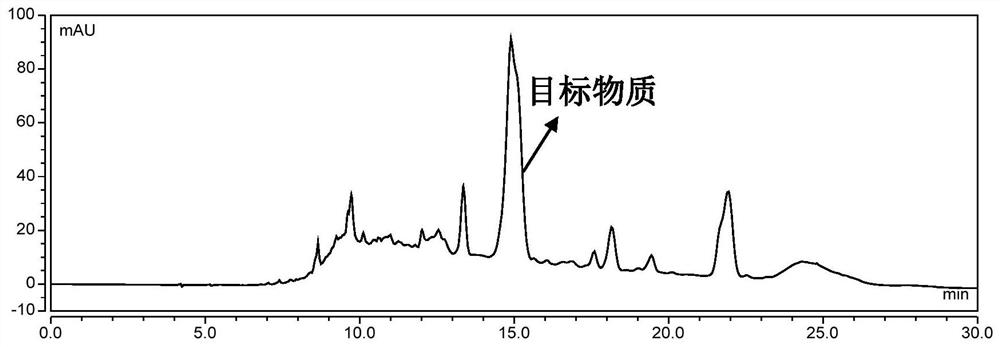
[0048] Put the D101 macroporous resin into the chromatographic column, and use ethanol, 0.5mol / L hydrochloric acid solution, 0.5mol / L sodium hydroxide solution, and water to wash in sequence, ...
Embodiment 2
[0052] Wash and peel the grapes to obtain 5kg of grape skins, add 70% ethanol solution containing 0.5% (v / v) hydrochloric acid according to the ratio of solid to liquid 1g: 6mL and mix thoroughly, ultrasonically extract for 90min, (control the temperature below 50°C , protected from light), filtered through gauze, the filtrate was centrifuged at 4000rpm for 10min, and the supernatant was taken. The filter residue was extracted once more in the same way. The filtrates were combined and filtered one more time using a Buchner funnel. The filtrate was evaporated under reduced pressure at 40°C to remove ethanol and concentrated to obtain a crude grape skin anthocyanin extract.
[0053] Put the AB-8 macroporous resin into the chromatographic column, and use ethanol, 0.5mol / L hydrochloric acid solution, 0.5mol / L sodium hydroxide solution, and water to wash in sequence, and then wash the crude anthocyanin extract at 0.2BV / The flow rate of h is injected into the chromatography colum...
Embodiment 3
[0057] Wash and peel the grapes to obtain 10kg of grape skins, add 60% ethanol solution containing 0.5% (v / v) hydrochloric acid according to the ratio of solid to liquid 1g: 5mL, fully mix, and ultrasonically extract for 60min, (control the temperature below 50°C , protected from light), filtered through gauze, the filtrate was centrifuged at 4000rpm for 10min, and the supernatant was taken. The filter residue was extracted once more in the same way. The filtrates were combined and filtered one more time using a Buchner funnel. The filtrate was evaporated under reduced pressure at 40°C to remove ethanol and concentrated to obtain a crude grape skin anthocyanin extract.
[0058] Put the AB-8 macroporous resin into the chromatographic column, and use ethanol, 0.5mol / L hydrochloric acid solution, 0.5mol / L sodium hydroxide solution, and water to wash in sequence, and then wash the crude anthocyanin extract at 0.2BV / The flow rate of h is injected into the chromatography column. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com