A kind of polymer based on phenazine trimer and its preparation method and battery application
A technology of phenazine trimer and polymer, which is applied in battery electrodes, circuits, electrical components, etc., can solve the problems of low actual capacity and dependence on scarce natural resources, and achieve reduced production costs, abundant material sources, and improved stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
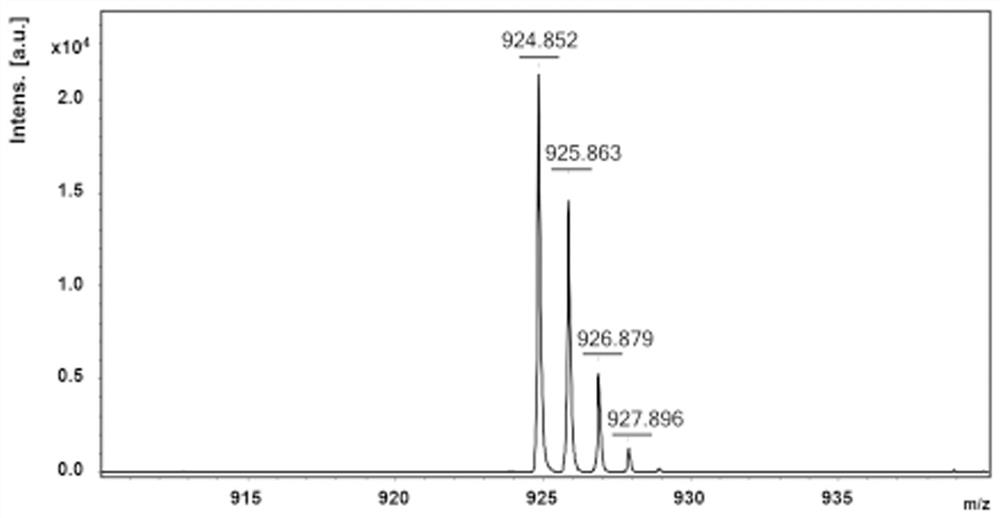
[0045] Example 1: Synthesis of p-TPZB1
[0046] 1.TPZB (X=Z=H, Y=CH=CH 2 )Synthesis
[0047]
[0048] 5,10-dihydrophenazine (5.47g, 30mmol), 1,3,5-tribromobenzene (3.15g, 10mmol), 4-bromostyrene (5.49g, 30mmol), palladium acetate (202mg, 1.8mmol ), xPhos (1.72g, 3.6mmol), sodium tert-butoxide (8.65g, 90mmol) and toluene (30ml) were put into the reaction flask, and oxygen was removed by freezing-vacuum-thawing cycle 3 times. The mixture was condensed under reflux at 100° C. and stirred for 12 hours. After the obtained crude product was cooled to room temperature, it was purified by column chromatography with neutral alumina (DCM:PE=1:2 to 1:1), and then recrystallized (mixture of DCM and MeOH). 1 H NMR (400MHz, C6D6, ppm) δ = 7.28 (d, J = 8.4Hz, 6H), 7.18 (d, J = 3.3Hz, 9H), 6.56 (dd, J = 17.6, 10.9Hz, 3H), 6.38 (dtd, J=23.1,7.5,1.4Hz,12H),6.15(dd,J=7.7,1.4Hz,6H),5.93(dd,J=7.8,1.4Hz,6H),5.61(d,J=17.5 Hz,3H),5.14(d,J=11.1Hz,3H).13C NMR(101MHz,C6D6,ppm)δ=146.37,139.71,137...
Embodiment 2
[0051] Embodiment 2: the synthesis of TPZB-6COOLi
[0052]
[0053] 1. Synthesis of TPZB-6COOMe
[0054] 5,10-dihydrophenazine (2.73g, 15mmol), 1,3,5-tribromobenzene (1.57g, 5mmol), dimethyl 5-bromo-isophthalate (4.1g, 15mmol), acetic acid Palladium (100mg, 0.9mmol), xPhos (860mg, 1.8mmol), cesium carbonate (14.7g, 90mmol) and . Toluene (30ml) was put into the reaction bottle, and the oxygen was removed by freezing-vacuum-thawing cycle 3 times. The mixture was refluxed and condensed at 130°C for 12 hours with stirring. The obtained crude product was quenched with methanol, cooled to room temperature, purified by column chromatography with neutral alumina (PE:EA=3:1), and recrystallized (mixture of DCM and MeOH). 1 H NMR (400MHz, C6D6, ppm) δ = 9.05 (t, J = 1.6Hz, 1H), 8.42 (d, J = 1.6Hz, 2H), 7.32 (s, 1H), 6.43 (td, J = 7.8, 1.3Hz, 3H), 6.31(td, J=7.8, 1.3Hz, 3H), 6.21(dd, J=7.9, 1.2Hz, 3H), 5.81(dd, J=7.9, 1.2Hz, 3H), 3.41( s,6H).
[0055] 2. Synthesis of TPZB-6COOLi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com