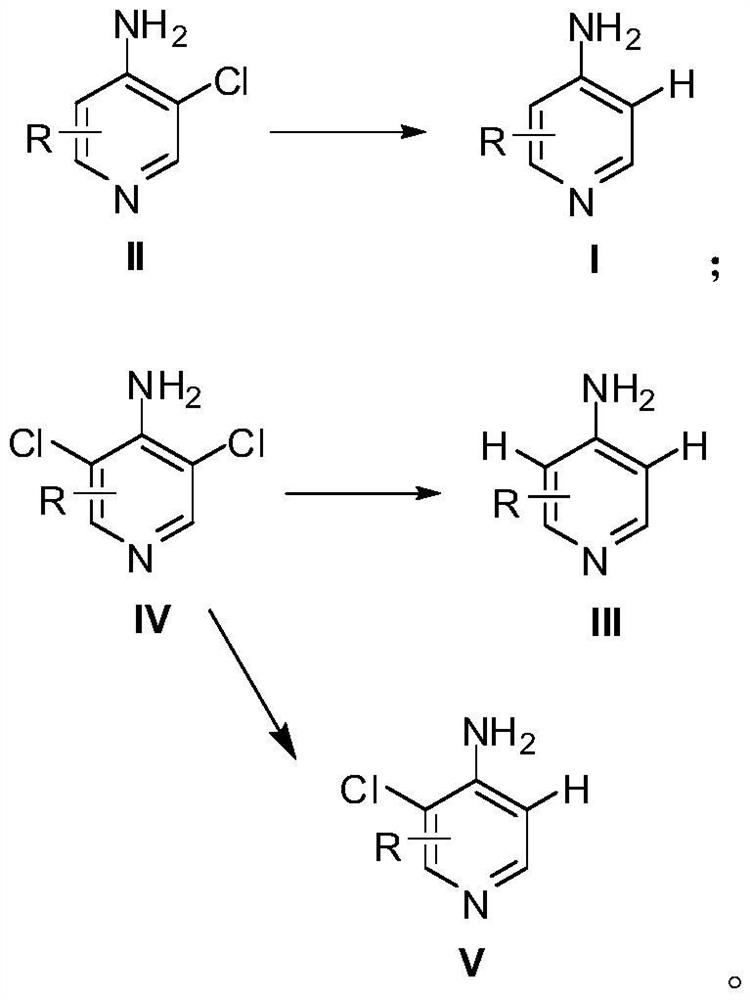
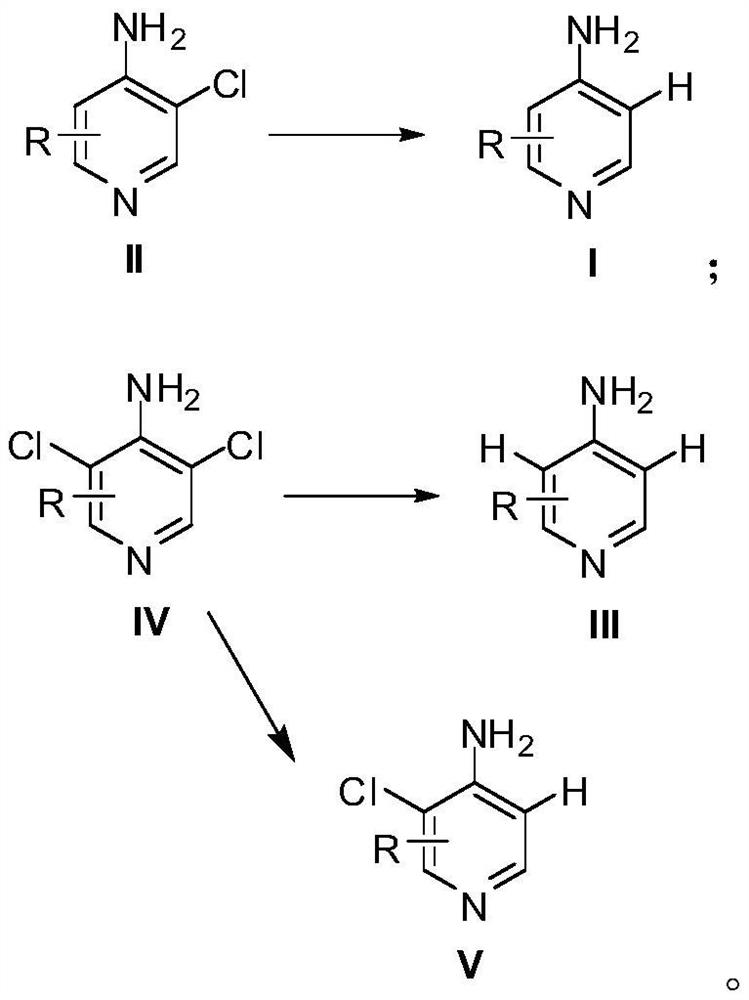
Preparation method of 4-aminopyridine compound
An aminopyridine and compound technology, applied in the field of organic compound preparation, can solve problems such as low preparation efficiency and complicated operation, and achieve the effects of simple preparation method, high reaction efficiency, good universality and tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation method of 4-amino-3-chloro-2,6-difluoropyridine
[0023] Synthesis of 3,5-dichloro-2,4,6-trifluoropyridine: Mix 250g of pentachloropyridine, 190g of anhydrous potassium fluoride and 600mL of sulfolane, stir and react at 150°C for 2h, cool down to room temperature, and monitor the raw materials by sampling After complete conversion, the reaction solution was poured into 3L of ice water and extracted with dichloromethane. The organic phase was washed with water and saturated sodium chloride solution successively, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The obtained crude product was purified by rectification to obtain 174.5 g of a colorless liquid, which was 3,5-dichloro-2,4,6- Trifluoropyridine, GC detection content 98.7%, yield 87%.
[0024] Synthesis of 4-amino-3,5-dichloro-2,6-difluoropyridine: Cool 500mL of 25% ammonia water to 0°C, slowly add 100g of 3,5-dichloro-2,4,6-tri Fluoropyridine, a white solid gradually pr...
Embodiment 2
[0027] The preparation method of 4-amino-2,6-difluoropyridine
[0028] Add 40g of 4-amino-3,5-dichloro-2,6-difluoropyridine, 86g of potassium phosphate, 4g of 10% palladium carbon catalyst (water content 54%) and 300mL of 50% ethanol aqueous solution into the hydrogenation kettle, and successively use nitrogen, Replace with hydrogen, then react under 0.5MPa hydrogen pressure at 65°C for 8 hours, cool down to room temperature, evaporate most of the solvent under reduced pressure, then add 150mL of water to dilute, filter with suction, wash the filter cake fully with water, and then recrystallize with 15mL of 50% ethanol aqueous solution , to obtain 24 g of white flaky solid, which is 4-amino-2,6-difluoropyridine, with an HPLC content of 98.5% and a yield of 92%.
Embodiment 3
[0030] Preparation method of 4-amino-2,5,6-trifluoropyridine
[0031] Synthesis of 3-chloro-2,4,5,6-tetrafluoropyridine: Mix 70g of 3,5-dichloro-2,4,6-trifluoropyridine, 22g of anhydrous potassium fluoride and 150mL of sulfolane at 180°C Stir the reaction for 2.5 h, lower to room temperature, take a sample to monitor the complete conversion of the raw material, pour the reaction solution into 1 L of ice water, and extract with dichloromethane. The organic phase was washed with water and saturated sodium chloride solution successively, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The obtained crude product was purified by rectification to obtain 46.5 g of a colorless liquid, which was 3-chloro-2,4,5,6-tetra Fluoropyridine, GC content 98.3%, yield 72%.
[0032] Synthesis of 4-amino 3-chloro-2,5,6-trifluoropyridine: Cool 200mL of 25% ammonia water to 0°C, slowly add 40g of 3-chloro-2,4,5,6-tetrafluoropyridine dropwise, A white solid gradually pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com