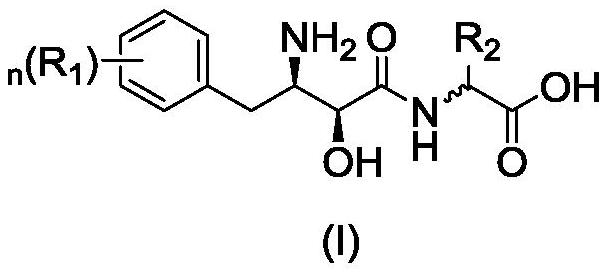
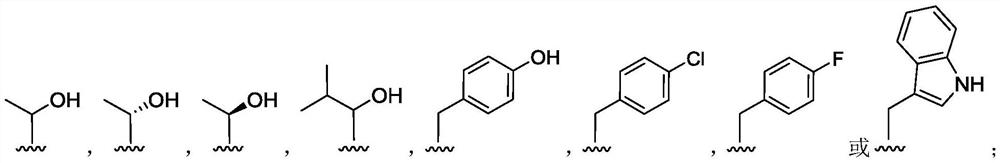
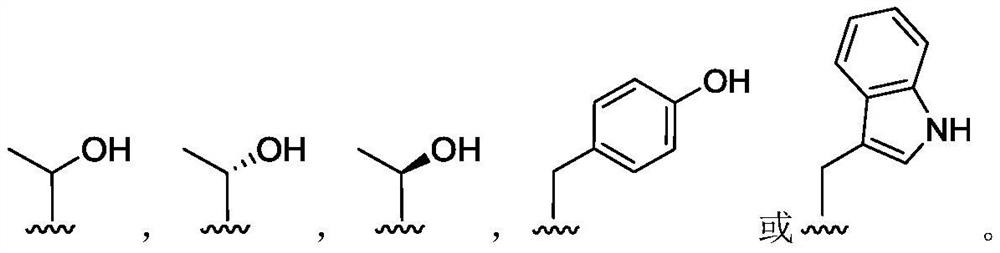
(2S, 3R)-3-amino-2-hydroxy-4-phenylbutyrylamide derivative as well as preparation method and application thereof
An amino and nitro group technology, which is applied in the preparation of carbamic acid derivatives, carboxylic acid amides, and cyanide reactions, etc., can solve the problems of no symptom-relieving drugs and no curable drugs for lymphedema.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the preparation of compound 1
[0049]
[0050] Preparation of intermediate 1b:
[0051]
[0052] Compound 1a (31g, 0.16mol) was dissolved in 310mL tetrahydrofuran / water (1:1) mixed solvent, triethylamine (32g, 0.32mol), di-tert-butyl dicarbonate (42g, 0.19mol) were added under ice-cooling ), react at room temperature after addition. After the reaction of the raw materials is complete, add water to quench the reaction under an ice-water bath, then add dilute hydrochloric acid to adjust the pH value to acidity, extract twice with ethyl acetate, combine the organic phases, wash with water once, and dry with anhydrous sodium sulfate. Suction filtration, the filtrate was concentrated to obtain 38g of intermediate 1b, yield: 81.0%;
[0053] ESI-MS:m / z=296(M+H) + .
[0054] Preparation of intermediate 1d:
[0055]
[0056] Compound 1c (1.2g, 10mmol) was dissolved in 10mL of methanol, thionyl chloride (5.95g, 50mmol) was added under ice-cooling, and...
Embodiment 2
[0070] Embodiment 2: the preparation of compound 2
[0071]
[0072] Preparation of Intermediate 2b:
[0073]
[0074] Compound 2a (1.2 g, 10 mmol) was dissolved in 10 mL of methanol, and thionyl chloride (5.95 g, 50 mmol) was added under ice-cooling, and reacted at room temperature after the addition was complete. After the raw materials reacted completely, the reaction system was concentrated to obtain 1.7 g of intermediate 2b, yield: 100%.
[0075] Preparation of Intermediate 2c:
[0076]
[0077] Intermediate 1b (2.2g, 7.46mmol), Intermediate 2b (1.39g, 8.2mmol) were dissolved in 20mL of dichloromethane, and triethylamine (3.03g, 30mmol), 1-hydroxybenzene Add triazole (1.2g, 8.95mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (1.72g, 8.95mmol), react at room temperature after addition. After the reaction of the raw materials is complete, concentrate, then add water and ethyl acetate to dissolve, adjust the pH to 5-6 with dilute hydrochloric a...
Embodiment 3
[0088] Embodiment 3: the preparation of compound 3
[0089]
[0090] Preparation of Intermediate 3b:
[0091]
[0092] Compound 3a (1.2 g, 10 mmol) was dissolved in 10 mL of methanol, and thionyl chloride (5.95 g, 50 mmol) was added under ice-cooling, and reacted at room temperature after the addition was complete. After the raw materials reacted completely, the reaction system was concentrated to obtain 1.7 g of intermediate 3b, yield: 100%.
[0093] Preparation of Intermediate 3c:
[0094]
[0095]Intermediate 1b (2.2g, 7.46mmol), intermediate 3b (1.39g, 8.2mmol) were dissolved in 20mL of dichloromethane, triethylamine (3.03g, 30mmol), 1-hydroxybenzene Add triazole (1.2g, 8.95mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (1.72g, 8.95mmol), react at room temperature after addition. After the reaction of the raw materials is complete, concentrate, then add water and ethyl acetate to dissolve, and adjust the pH to 5-6 with dilute hydrochloric ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com