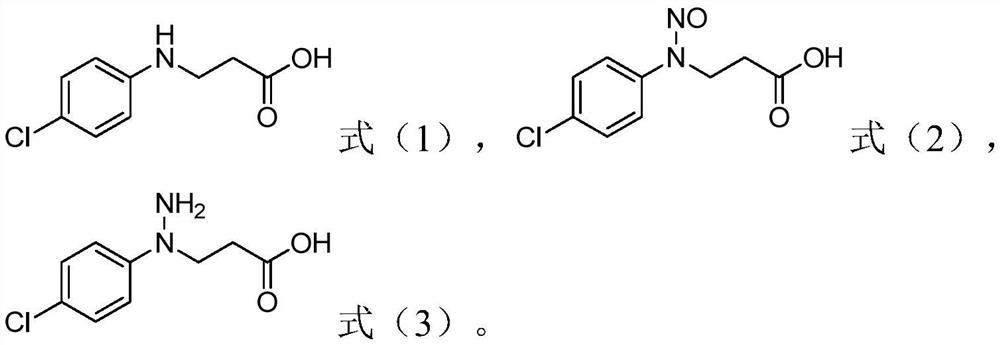
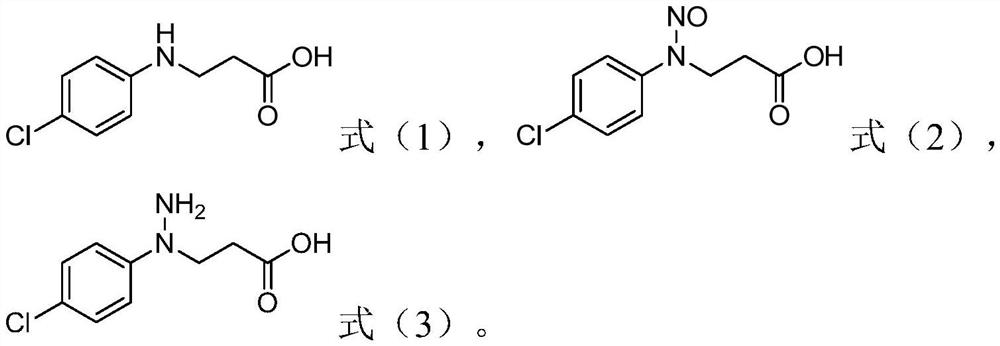
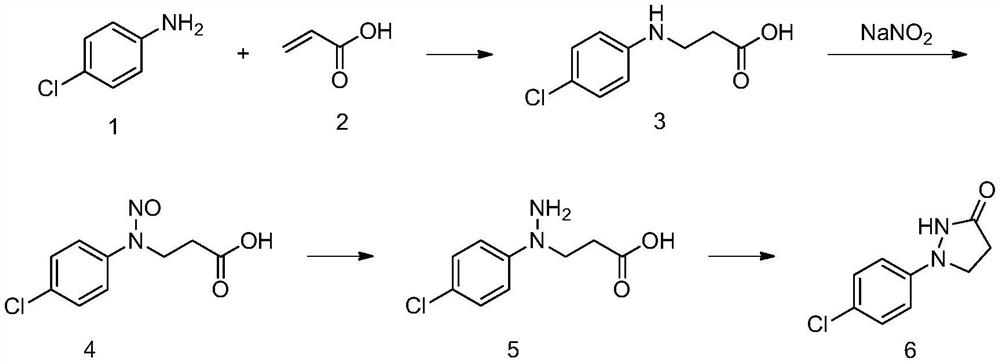
Process for the preparation of 1-(4-chlorophenyl)-pyrazolidin-3-ones
A technology of pyrazolidine and chlorophenyl, applied in the field of fungicides synthesis, can solve the problems of high safety risk, small amount of three wastes, large amount of three wastes, etc., and achieves the effects of high reaction safety and small amount of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] 1) Synthesis of compound 3
[0067] Add 25.7g of p-chloroaniline 1 and 100mL of toluene into the reaction bottle, raise the temperature to 80°C, add 16.0g of acrylic acid 2 dropwise, and continue to keep warm for 3 hours to complete the reaction. The reaction solution is directly used in the next reaction, and the reaction yield is 95% %. In addition, after the completion of the reaction, a sample was taken for mass spectrometry, and the results are as follows.
[0068] MS m / z: 200 (M+1).
[0069] 2) Synthesis of Compound 4
[0070] In the toluene solution containing 37.8g compound 3, add 27.7g content and be 30% by weight of hydrochloric acid, control temperature at 5-10 ℃, add dropwise the aqueous solution containing 13.9g sodium nitrite (sodium nitrite content is 33% by weight), After the dropwise addition was completed and the reaction was continued for 0.5 hours, the aqueous phase was separated, and the organic phase was directly used for the next reaction, and ...
Embodiment 2
[0080] 1) Synthesis of compound 3
[0081] Add 25.7g of p-chloroaniline 1 and 13mL of chlorobenzene into the reaction flask, keep the temperature at 20°C, add 21.8g of acrylic acid 2 dropwise, and continue to keep warm for 24 hours to complete the reaction. Rate 90%. In addition, after the completion of the reaction, a sample was taken for mass spectrometry, and the results are as follows.
[0082] MS m / z: 200 (M+1).
[0083] 2) Synthesis of Compound 4
[0084] In the chlorobenzene solution that contains 35.8g compound 3, add 26.3g content and be 30% by weight hydrochloric acid, control temperature at minus 10-0 ℃, add dropwise the aqueous solution that contains 15.1g sodium nitrite (sodium nitrite content is 35% by weight ), after the dropwise addition was completed and the insulation reaction was continued for 0.5 hours, the aqueous phase was separated, and the organic phase was directly used for the next step reaction, and the reaction yield was 95%. In addition, the or...
Embodiment 3
[0092] 1) Synthesis of compound 3
[0093] Add 25.7g of p-chloroaniline 1 and 257mL of dichloroethane into the reaction flask, raise the temperature to reflux, add 14.7g of acrylic acid 2 dropwise, and continue the heat preservation reaction for 1 hour to complete the reaction. The reaction solution is directly used in the next step reaction. Rate 90%. In addition, after the completion of the reaction, a sample was taken for mass spectrometry, and the results are as follows.
[0094] MS m / z: 200 (M+1).
[0095] 2) Synthesis of Compound 4
[0096] In the ethylene dichloride solution that contains 35.8g compound 3, add 26.3g content and be the hydrochloric acid of 30% by weight, control temperature at 10-20 ℃, add dropwise the aqueous solution that contains 16.9g sodium nitrite (sodium nitrite content is 20% by weight %), after the dropwise addition was completed and the insulation reaction was continued for 0.5 hours, the aqueous phase was separated, and the organic phase wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com