Nosiheptide flavored chewable tablets, preparation method thereof and application of nosiheptide-flavored chewable tablets in clostridium welchii disease
A technology of nosiheptide and chewable tablets, which is applied in the field of nosiheptide flavored chewable tablets and its preparation, which can solve the problems of the stability of main ingredients, disintegration of tablets, and increased dissolution, so as to improve solubility and bioavailability, Effects of improving palatability and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] A preparation method of nosiheptide flavor chewable tablets, comprising the steps of:
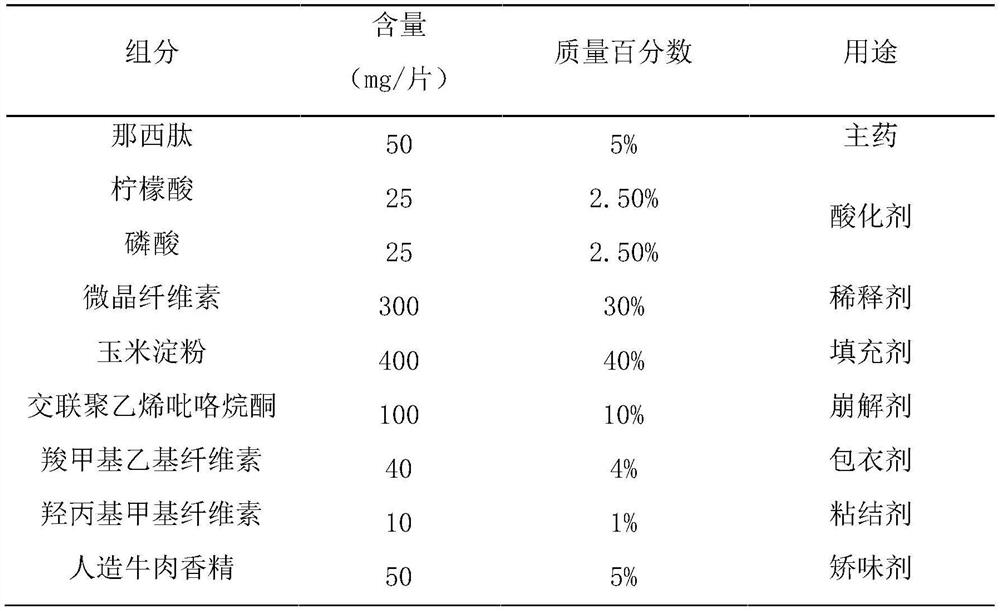
[0042] Step 1, prepare materials according to the formula, the formula includes: nosiheptide 1%-10%, acidulant 5%, diluent 30%-40%, binder 1%, filler 30%-40%, Disintegrant 2%-10%, flavoring agent 5%-20%, coating agent 1%-5%;
[0043] Step 2, crushing Nosiheptide, diluent, filler, disintegrating agent, binder, and flavoring agent, and then sieving and filtering respectively; as a preferred method, 80-mesh sieve is used for sieving.
[0044] Step 3: Mix nosiheptide, diluent, and disintegrant to obtain a mixture, prepare a binder and an acidifier together to obtain a binding liquid, mix the mixture and the binding liquid to form a soft material, and sieve to form granules. After drying at 40°C-45°C, sieve to obtain granules; as a preferred method, pass through a 40-mesh sieve to make granules, dry at 40°C-45°C, and then pass through 40-mesh and 65-mesh sieves for granulation, remove C...
Embodiment 1
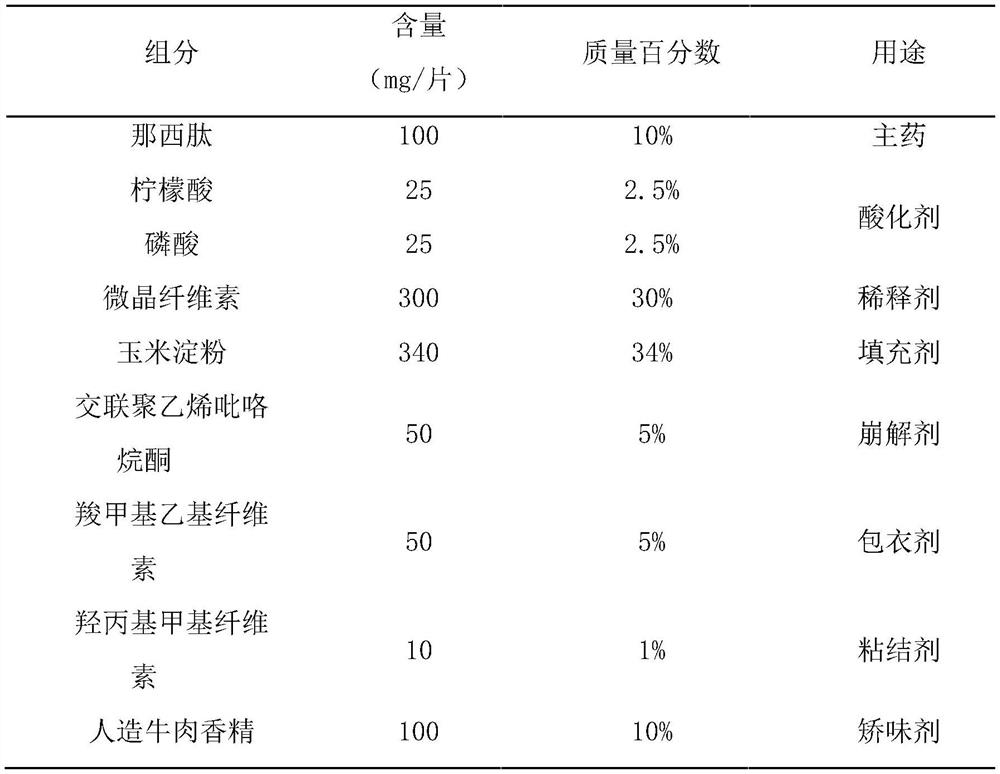
[0056] This example prepares nosiheptide flavored chewable tablets of the present invention, which are made of raw materials with mass percentages as in Table 1:
[0057] Table 1 Each component and its content of nosiheptide flavor chewable tablet of the present invention
[0058]
[0059] Preparation method: pass nosiheptide, microcrystalline cellulose, corn starch, cross-linked polyvinylpyrrolidone and artificial beef essence through 80-mesh sieve respectively, and take nosiheptide, microcrystalline cellulose, cross-linked Polyvinylpyrrolidone, mixed evenly, added the previously prepared binding solution A, made soft material, passed through a 40 mesh sieve to granulate, dried at 45°C, passed through a 40 mesh and 65 mesh sieve for granulation, and removed coarse particles and Fine powder to obtain granules; the above-mentioned granules are fluidized bed coated with the coating solution prepared in advance, and the weight of the coating is increased by 5%, and then passed...
Embodiment 2
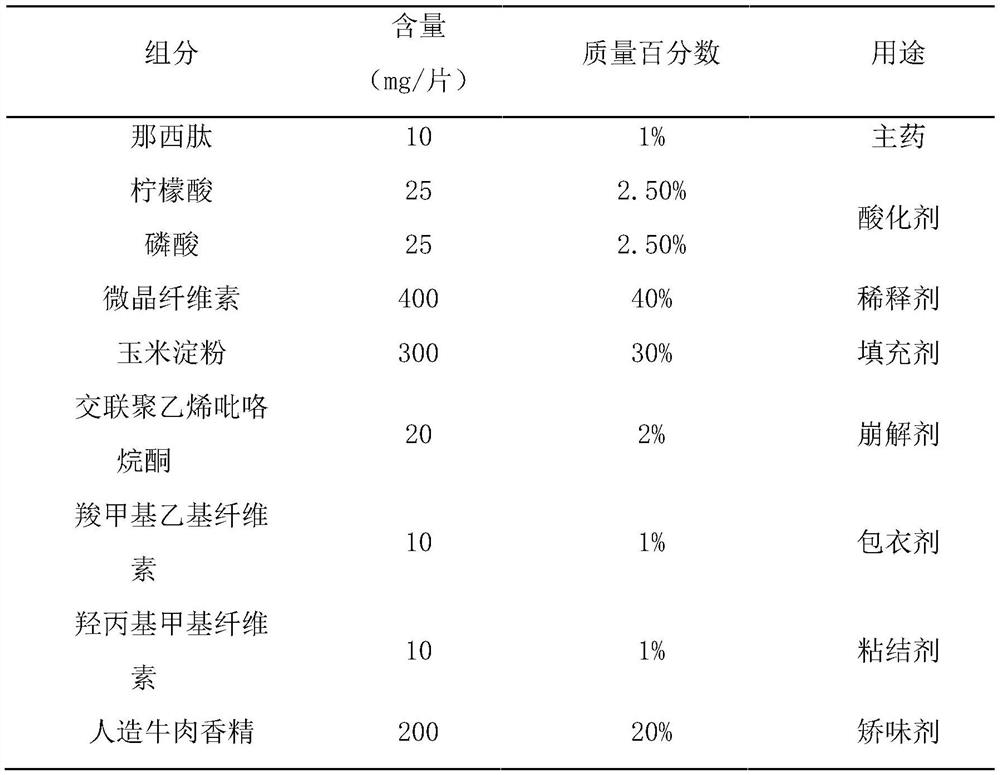
[0061] This embodiment prepares nosiheptide flavored chewable tablets of the present invention, which are made of raw materials with mass percentages as in Table 2:
[0062] Table 2 each component and its content of nosiheptide flavor chewable tablet of the present invention
[0063]
[0064] Preparation method: pass nosiheptide, microcrystalline cellulose, corn starch, cross-linked polyvinylpyrrolidone and artificial beef essence through 80-mesh sieve respectively, and take nosiheptide, microcrystalline cellulose, cross-linked Polyvinylpyrrolidone, mixed evenly, added the previously prepared binding solution A, made soft material, passed through a 40 mesh sieve to granulate, dried at 45°C, passed through a 40 mesh and 65 mesh sieve for granulation, and removed coarse particles and Fine powder to obtain granules; the above-mentioned granules are fluidized bed coated with the coating solution prepared in advance, and the weight of the coating is increased by 5%, and then pas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com