Carborane celecoxib, preparation thereof and application thereof in head and neck cancer boron neutron capture therapy drugs
A carboryl plug and coxib technology, which is applied in the fields of medicinal chemistry and radiation medicine, can solve the problems of tumor targeting and low boron content, achieve good inhibitory effect, strong selective uptake, and reduce side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
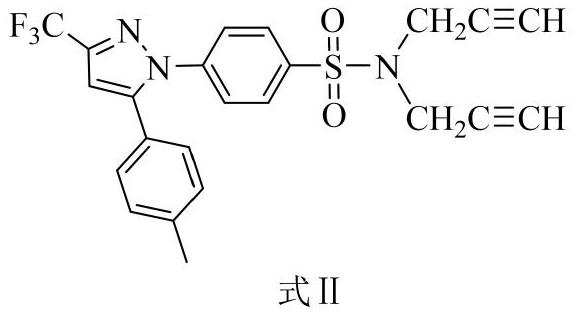
[0027] Embodiment 1: the synthesis of intermediate product formula II
[0028]
[0029] Use celecoxib as the reactant and 3-bromopropyne for substitution reaction in an acetone solution dissolved in potassium carbonate, wherein the molar ratio of celecoxib, potassium carbonate and 3-bromopropyne is 1:2.5:2.3 , the organic solvent consumption is 200 times of the molar number of celecoxib. After the substitution reaction, the filtrate was collected by filtration, and the solvent was removed under reduced pressure to obtain the crude product of celecoxib derivative, and then the crude product of celecoxib derivative was passed through petroleum ether and ethyl acetate in a volume ratio of 10:1 to 7:1. The high-purity intermediate product can be obtained by silica gel column chromatography separation with ester eluent.
[0030] The detection data of the product are as follows: white solid, yield 86.0%. 1 H NMR (400MHz, Chloroform-d, ppm): δ7.74(d, J=8.7Hz, 2H), 7.39(d, J=8.6H...
Embodiment 2
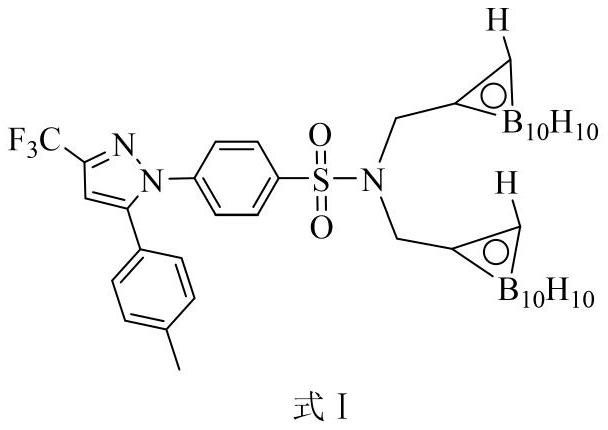
[0031] Embodiment 2: Synthesis of carboryl celecoxib formula I
[0032]
[0033] Decaborane and acetonitrile react in toluene solution at 110°C to form decaborane acetonitrile complex, and then decaborane acetonitrile complex is heated with N,N-bis(propynyl)-celecoxib in toluene solution Addition reaction occurs at 110°C, wherein the molar ratio of decaborane to N,N-bis(propynyl)-celecoxib is 3:1, and the amount of organic solvent acetonitrile and toluene is the number of moles of decaborane 100 times and 400 times. After the addition reaction, remove the solvent under reduced pressure to obtain the crude product of celecoxibated carborane, and then pass the crude product of celecoxibated carborane through n-hexane and acetic acid with a volume ratio of 15:1 to 10:1 Carboryl celecoxib with high purity can be obtained by silica gel column chromatography separation with ethyl ester eluent.
[0034] The detection data of the product are as follows: white solid, yield 75.0%. ...
Embodiment 3
[0035] Example 3: Uptake detection of carboryl celecoxib in CAL27 cells
[0036] CAL27 cells in the logarithmic growth phase with a cell content of 80-85% were seeded into 6-well plates (1×10 per well 6cells). After the cells adhered to the wall, the control group (BSH or celecoxib) or carboryl celecoxib with a concentration of 20, 50 or 100 μM was used to act on the cells for 24 hours respectively (4 parallel experiments were set up for each group, and Set up a blank control group). After the effect, the culture medium was discarded, and the cells were washed 3 times with PBS; then 1 mL of pronase solution with a concentration of 2 mg / mL was added to each well and incubated at 4 °C for 4 h to remove the compound bound to the cell surface. The pronase-containing solution was discarded, the cells were washed 3 times with PBS, the cells were lysed with RIPA lysate, the cell lysate was collected, and the supernatant was collected by centrifugation at 10,000 rpm. The supernatan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com