Cytochrome P450 enzyme mutant and application thereof
A cytochrome, mutant technology, applied in applications, enzymes, bacteria, etc., to achieve the effect of improving activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] In a typical embodiment of the present invention, there is also provided a method for preparing a carbonyl compound or an alcohol compound, the preparation method comprising: using any of the above-mentioned cytochrome P450 enzyme mutants to catalyze the alkene compound Carries out direct reverse Markov oxidation to carbonyl compounds or alcohol compounds , wherein R represents an optionally substituted or unsubstituted alkyl group, an optionally substituted or unsubstituted aralkyl group, or an optionally substituted or unsubstituted aryl group.
[0052] Preferably, R represents an optionally substituted or unsubstituted alkyl group, an optionally substituted or unsubstituted aralkyl group, or an optionally substituted or unsubstituted aryl group with 1-20 carbon atoms, more preferably Yes, R represents an optionally substituted or unsubstituted alkyl group, an optionally substituted or unsubstituted aralkyl group, or an optionally substituted or unsubstituted aryl...
Embodiment 1
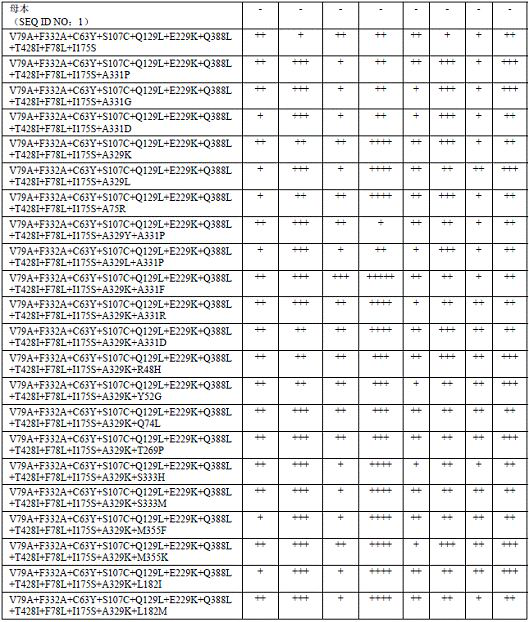
[0066] Add 1.5 mM raw material 1, raw material 2, raw material 3 and raw material 4 to a 10 mL glass bottle, add 1 eq NADP + , 20eq glucose, 3 wt glucose dehydrogenase, 0.1 g P450 enzyme, make up to 4 mL with Tris-HCl buffer (pH 8.0, 100 mM), mix well, and react at 30°C, 200 rpm shaker for 3h. After the reaction, add 2 mL of ethyl acetate, mix well and then centrifuge at 12,000 rpm for 5 min, take the upper layer, and detect it by HPLC with a wavelength of 210 nm. The response characteristics of some mutants are shown in Table 1.
[0067] Table 1:
[0068]
[0069] The above female parent is SEQ ID NO: 1; the multiples of activity and selectivity improvement are represented by +, + means an increase of 0-1 times, ++ means an increase of 1-2 times, +++ means an increase of 2-3 times, ++ ++ indicates an increase of 3-5 times, and +++++ indicates an increase of 5-10 times. Selectivity in the present invention / selectivity of anti-Markov oxidation is defined as: aldehyde prod...
Embodiment 2
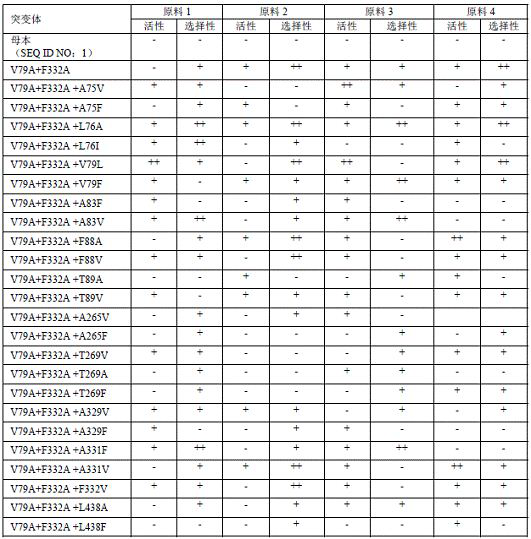
[0071] Add 1.5 mM raw material 1, raw material 2, raw material 3 and raw material 4 to a 10 mL glass bottle, add 1 eq NADP + , 20eq glucose, 3 wt glucose dehydrogenase, 0.1 g P450 enzyme, make up to 4 mL with Tris-HCl buffer (pH 8.0, 100 mM), mix well, and react at 30°C, 200 rpm shaker for 3h. After the reaction, add 2 mL of ethyl acetate, mix well and then centrifuge at 12,000 rpm for 5 min, take the upper layer, and detect it by HPLC with a wavelength of 210 nm. The response characteristics of some mutants are shown in Table 2.
[0072] Table 2:
[0073]
[0074] The above female parent is SEQ ID NO: 1; the multiples of activity and selectivity improvement are represented by +, + means an increase of 0-1 times, ++ means an increase of 1-2 times, +++ means an increase of 2-3 times, ++ ++ indicates an increase of 3-5 times, and +++++ indicates an increase of 5-10 times.
[0075] It can be seen from the results in Table 2 that the directed evolution method using random mu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com