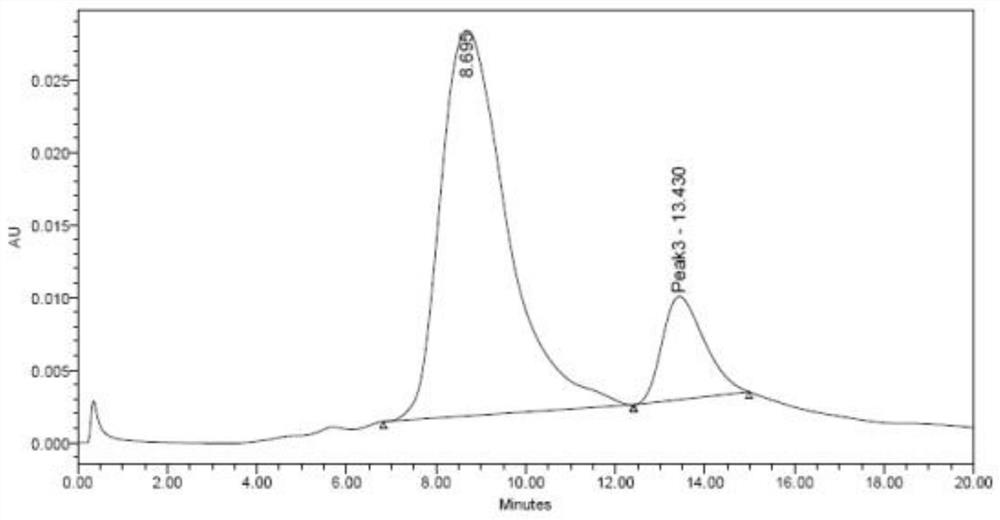
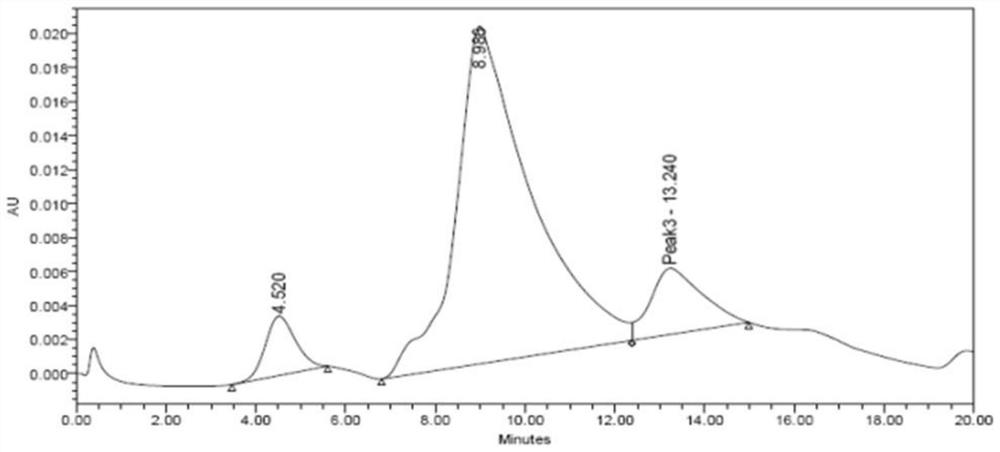
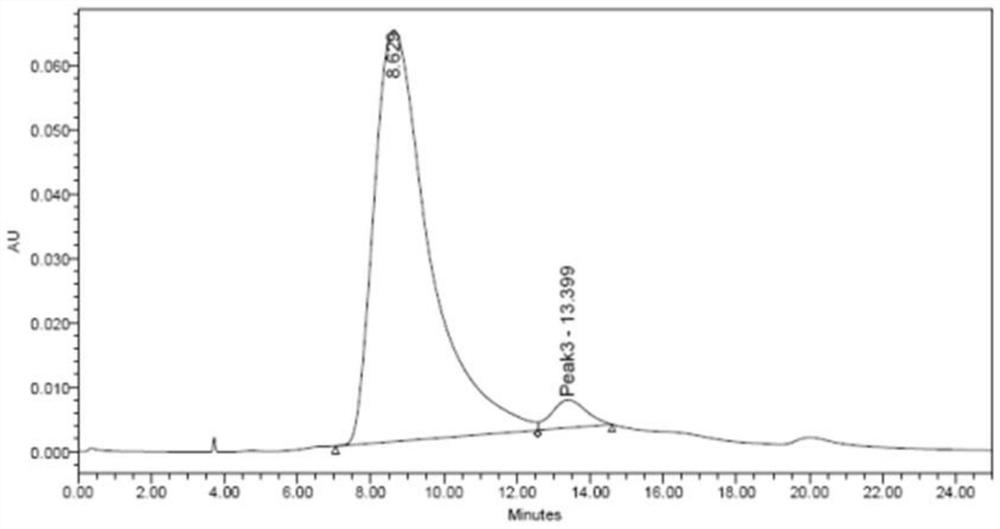
Polymer microneedle for inhibiting cell inflammatory factors to treat acute attack of gout and preparation method of polymer microneedle
A technology for acute attack of gout and inhibition of inflammation, applied in the directions of microneedling, chemical instruments and methods, needles, etc., can solve the problems of not being suitable for preventing attacks, limiting the effect of drugs, short biological half-life, etc., to reduce the phenomenon of poisoning, prolong the Release time, the effect of improving the absorption rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0057] 1. Preparation of pharmaceutical preparations
[0058] The pharmaceutical preparation of this example contains 0.005-0.05 parts by weight of canakinumab, 0.05-0.15 parts by weight of sodium phosphate, 0.0025-0.025 parts by weight of mannitol and 0.005-0.025 parts by weight of sodium chloride. In this example, the drug preparation was made into nanoparticles in advance, and then used in subsequent tests. The specific preparation method is as follows:
[0059] Weigh 0.05g of canakinumab and disperse it in the sodium phosphate buffer solution of pH7.1-9.0, add a small amount of polylactic acid-glycolic acid copolymer solution, the amount of polylactic acid-glycolic acid copolymer is about the weight of canakinumab 20%-70%, using a spray dryer to make the first nano particles. The sodium phosphate buffer contains 0.15 g of sodium phosphate.
[0060] Take by weighing 0.025g of sodium chloride and 0.025g of mannitol and mix evenly, and add in the polylactic acid-glycolic ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com