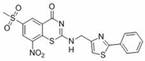
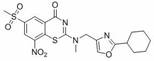
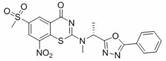
Benzothiazinone compound, preparation method thereof and application of benzothiazinone compound as antituberculosis drug
A technology of benzothiazinone and compounds, which is applied in the field of benzothiazinone compounds and their preparation and application as anti-tuberculosis drugs, can solve the problems of poor druggability, etc., and achieve low cLogP value, good druggability, and excellent inhibition effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of benzothiazinone compound of the present invention is as follows:
[0028] (1) Compound A5 is reacted with an amine compound to obtain a benzothiazinone compound;
[0029] (2) Reducing the benzothiazinone compound prepared in step (1) to obtain compound A7, which undergoes a substitution reaction to obtain the benzothiazinone compound;
[0030] Alternatively, the benzothiazinone compound prepared in step (1) is reduced to obtain compound A7, and the compound A7 undergoes a substitution reaction followed by an azide reaction to obtain a benzothiazinone compound;
[0031] Alternatively, the benzothiazinone compound prepared in step (1) undergoes an azide reaction to obtain the benzothiazinone compound.
[0032] Further, compound A3 is chlorinated to obtain compound A4; compound A4 is reacted with isothiocyanate to obtain compound A5; A4 to A6 are one-pot reactions.
[0033] Specifically, the preparation of each compound of the present invention ...
Embodiment 1
[0044] Example 1 Compound 1: 2-(4-(cyclohexylmethyl)piperazin-1-yl)-6-(methylsulfonyl)-8-nitro-4 H -Benzo[ e ][1,3]thiazin-4-one
[0045]
[0046] Dissolve ammonium thiocyanate (46 mg, 1.2 eq.) in 5 mL of anhydrous acetone, add polyethylene glycol (0.05 eq, based on compound A3) dropwise, and stir until dissolved at room temperature to obtain isothiocyanate ammonium solution;
[0047] Compound A3 (50 mg, 1eq, R 3 is methyl) dissolved in dichloromethane (anhydrous) (5 mL), dropwise N, N -Dimethylformamide (0.05eq., based on compound A3), add dropwise oxalyl chloride (0.25 mL, 2.5eq.), stir at room temperature for 0.5 hours after the addition is complete, spin dry the solvent and excess oxalyl chloride to obtain the corresponding intermediate acid chloride compound (compound A4, R 3 is methyl); then dropwise add the above-mentioned ammonium isothiocyanate solution, and stir at room temperature for 20 minutes after the dropwise addition is completed, to obtain intermedia...
Embodiment 2
[0049] Example 2 2-(Benzyl(methyl)amino)-6-(methylsulfonyl)-8-nitro-4 H -Benzo[ e ][1,3]thiazin-4-one
[0050]
[0051] Compared with Example 1, the amine used is N -Methyl-1-phenylmethylamine, the rest unchanged, to obtain compound 2 (2-(benzyl(methyl)amino)-6-(methylsulfonyl)-8-nitro-4 H -Benzo[ e ][1,3]thiazin-4-one) as a yellow solid (52% yield).
[0052] 1 H NMR (400 MHz, CDCl 3 ) δ 9.34 (s, 1H), 9.04 (s, 1H), 7.33-7.26 (m,5H), 5.13 – 4.94 (m, 2H), 3.40-3.29 (m, 3H), 3.19 (s, 3H). 13C NMR (151 MHz, CDCl 3 ) δ 165.8, 158.7, 144.0, 140.0, 136.0, 135.0, 129.3, 129.0, 128.3, 127.6, 127.1, 126.9, 54.6, 44.2, 30.0. MS-ESI (m / z): 405.7 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com