Synthetic method of octafluorotoluene
A technology of octafluorotoluene and synthesis method, applied in chemical instruments and methods, organic chemistry, preparation of halogenated hydrocarbons, etc., can solve the problems of difficult tar handling, high fluorination temperature, many side reactions, etc., and achieves the production of less tar, Fewer side reactions and the effect of lowering the reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
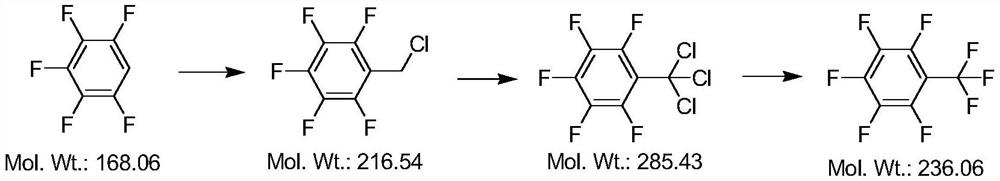
[0026] First step response:
[0027] Take a 3000mL four-neck flask with stirring, thermometer, and reflux condenser. Add 312g of 98% sulfuric acid, slowly add 72g of water, and lower the temperature to 0-5°C. Add 1000 g of pentafluorobenzene and 357 g of paraformaldehyde. 1160 g of chlorosulfonic acid was added dropwise. After the dropwise addition, the temperature was raised to 45° C., and a sample was taken after reacting for 2 hours. The raw material trifluoromethylbenzene was ≤10%. The temperature is lowered and stratified, the upper layer is the organic phase, and the lower layer is the waste concentrated sulfuric acid. Add 1000 g of water to the upper layer, stir, wash with water, and separate layers.
[0028] The upper layer is the aqueous phase, and the lower layer is the organic phase. Add 5% sodium hydroxide solution to the lower organic phase to adjust the pH to neutral. The layers are separated, and the lower layer is transferred to a still pot for rectificat...
Embodiment 2
[0034] First step response:
[0035] Take a 3000mL four-neck flask with stirring, thermometer, and reflux condenser. Add 312g of 98% sulfuric acid, slowly add 72g of water, and lower the temperature to 0-5°C. Add 1000 g of pentafluorobenzene and 320 g of paraformaldehyde. 1044 g of chlorosulfonic acid was added dropwise. After the dropwise addition, the temperature was raised to 35° C., and a sample was taken after reacting for 2 hours. The raw material trifluoromethylbenzene was ≤10%. Cooling and layering, adding 1000g of water to the upper layer, washing with water, and layering. Add 5% sodium hydroxide solution to the lower organic phase to adjust the pH to neutral. The layers are separated, and the lower layer is transferred to a still pot for rectification. The distillate before rectification is the raw material pentafluorobenzene, which is recovered for the next batch of reactions. At an oil temperature of 150°C, a top temperature of 120°C, and a pressure of -0.085...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com