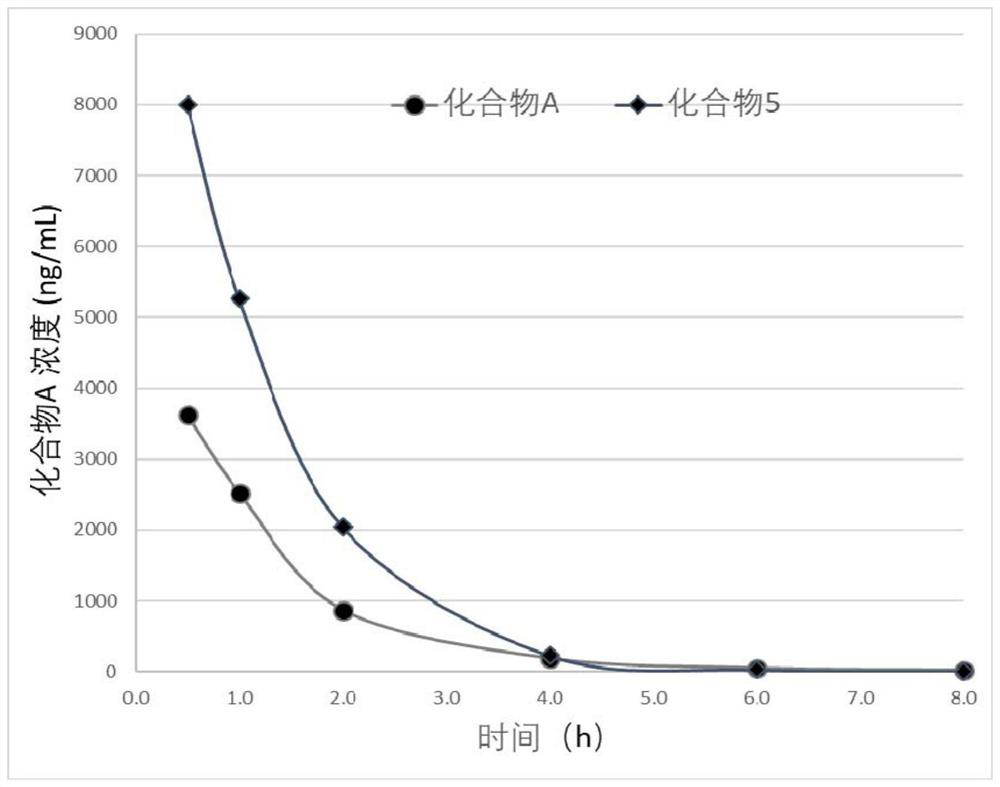
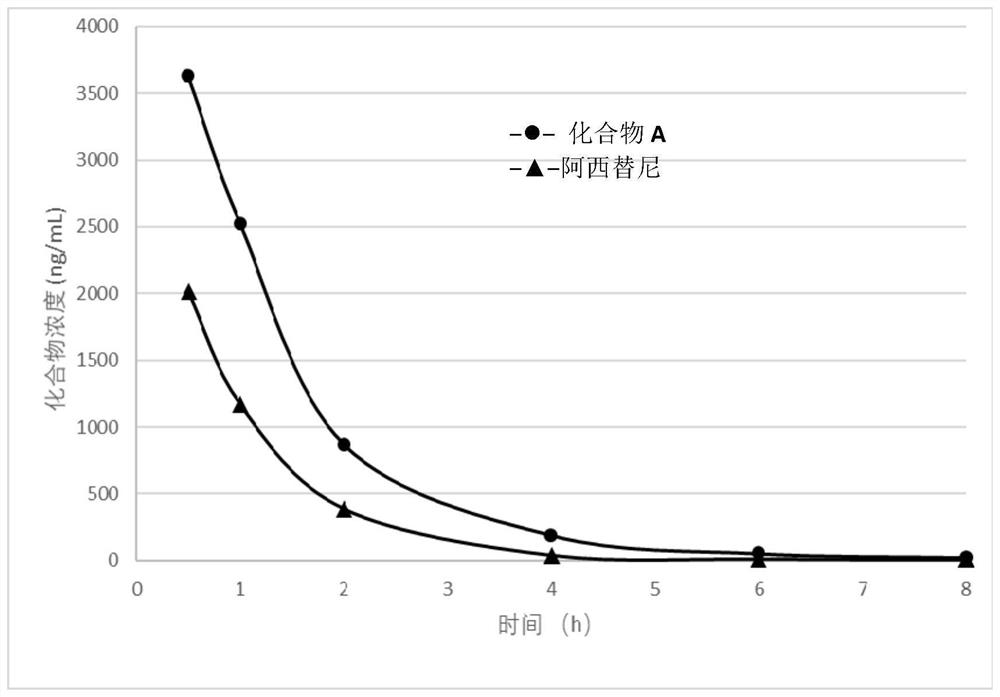
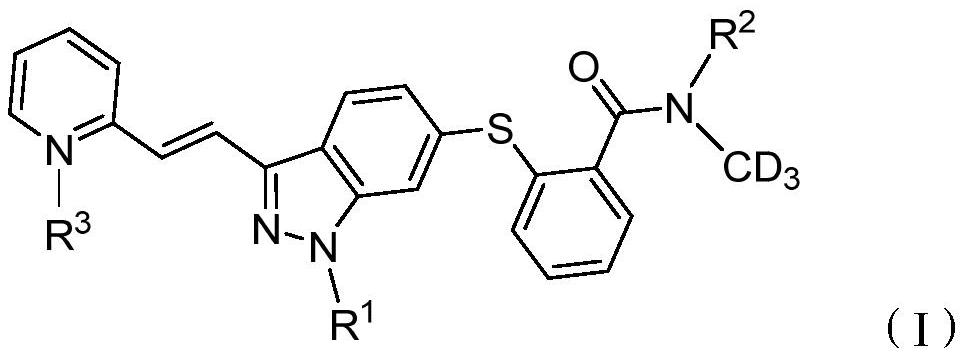
Deuterated compound and application thereof in treatment of cancer
A compound and hydrate technology, applied in the field of deuterated compounds and their application in the treatment of cancer, can solve problems such as explaining or explaining the chemical properties and biological activities of deuterium-enriched axitinib
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Example 1: N-(trideuteromethyl)-2-((3-((1E)-2-(2-pyridyl)vinyl)-1H-indazol-6-yl)thio)benzene Formamide (compound A) preparation
[0130] Aqueous NaOH solution (NaOH, 83.2 g, 2.08 mmol, 5.0 eq.; water, 132 mL) was placed in the reaction flask and cooled to 0 °C. At 0°C, first add CD to the reaction flask 3 OD (15g, 0.416mol, 1.0eq.), then a solution of TsCl in THF (TsCl, 95g, 0.500mol, 1.2eq.; THF, 132mL) was slowly added dropwise. After the dropwise addition, the temperature of the system was raised to room temperature, and the reaction mixture was continuously stirred at room temperature for 16 h. Then acetic acid (94.5 g) was added dropwise at 25° C. to neutralize, and filtered. The filtrate was extracted twice with ethyl acetate (200 mL each), the filter cake was dissolved in water (200 mL), and extracted twice with ethyl acetate (200 mL each), and the organic phases were combined. The organic phase was saturated with Na 2 CO 3 solution (300mL) after washing, w...
Embodiment 2
[0136] Example 2: N-(trideuteromethyl)-2-((3-((1E)-2-(2-pyridyl)vinyl)-1-(2,5,8,11-tetraoxo Preparation of heterododecanoyl)-1H-indazol-6-yl)thio)benzamide (compound 5)
[0137] Add triethylene glycol monomethyl ether (500mg, 3.045mmol, 1.0eq.), tetrahydrofuran (10mL) and triethylamine (616mg, 6.09mmol, 2.0eq.) successively in the reaction flask, and place the mixture in an ice-water bath Cool down to 0°C. Then, a tetrahydrofuran solution of phenyl p-nitrochloroformate (675 mg dissolved in 10 mL tetrahydrofuran, 3.350 mmol, 1.1 eq.) was added dropwise into the reaction system. Warm up to room temperature and stir for 5 hours. The reaction was monitored by TLC until the starting material was consumed. Concentrate to remove most of the solvent, then add more water (40 mL) and ethyl acetate (40 mL). The layers were separated by extraction and washing, and the organic phase was separated. The organic phase was concentrated, and the residue was separated and purified by silica...
Embodiment 3
[0139] Example 3: N-(trideuteromethyl)-2-((3-((1E)-2-(2-pyridyl)vinyl)-1-((1-naphthyloxy)((1S )-(1-methoxycarbonylethyl)amino)phosphinoyl)-1H-indazol-6-yl)thio)benzamide (compound 10)
[0140] Naphthol (720 mg, 4.99 mmol, 1.0 eq.) and diethyl ether (20 mL) were added to a reaction vial. Under the protection of nitrogen, the reaction system was placed under the cooling condition of -78 ° C, and phosphorus oxychloride (765 mg, 4.99 mmol, 1.0 eq.) and triethylamine (504 mg, 4.99 mmol, 1.0 eq.) were added dropwise to the above solution. ). The reaction mixture was stirred at -78°C for 1 hour, then slowly warmed to room temperature and stirred overnight. The insoluble matter was removed by filtration, and the filtrate was concentrated to obtain (1-naphthyloxy)phosphoryl dichloride (1.2 g, 92%). 1 H NMR (500MHz, CDCl 3 ): δppm 7.47(t, J=8.0Hz, 1H), 7.53-7.68(m, 3H), 7.82(d, J=8.0Hz, 1H), 7.91(d, J=7.7Hz, 1H), 8.10( d, J=7.9Hz, 1H).
[0141] Add (1-naphthyloxy)phosphoryl dichlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com