Preparation method of lithium difluoroborate
A technology of lithium difluorooxalate borate and lithium tetrafluoroborate is applied in chemical instruments and methods, compounds containing elements of Group 3/13 of the periodic table, organic chemistry, etc., and can solve problems such as poor quality of lithium difluorooxalate borate , to achieve the effect of improving the purity, reducing the production cost, and the preparation process is simple and safe.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
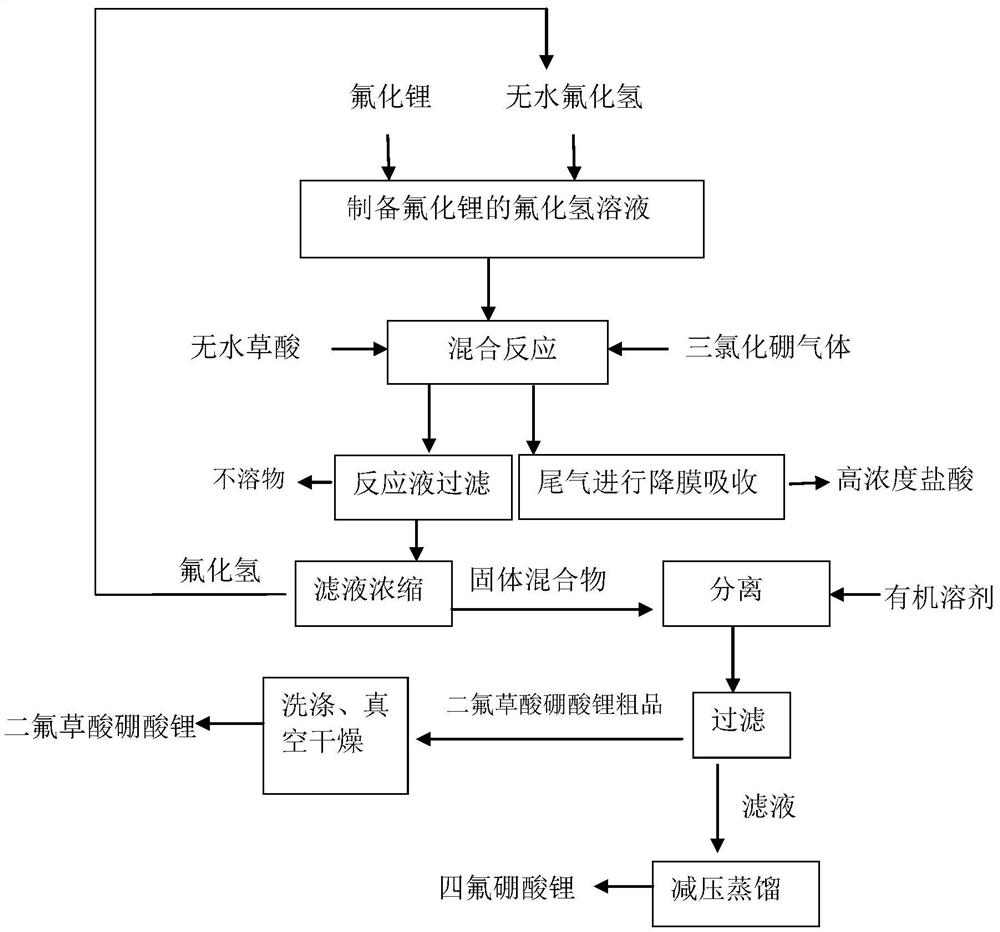
[0024]The preparation process of lithium boric acid acid salted acid salted acid isfigure 1 As shown, specifically includes the following steps:
[0025](1) in 1#In the reactor, 36.1 g of lithium fluoride (1.39 mol) was reactive with 556 g of hydrogen fluoride (27.8 mol) at a temperature of -40 to -30 ° C for 4 h, and the hydrogen fluoride solution of lithium fluoride was degraded.
[0026](2) Enter the fluoride solution of fluoride into 2#In the reactor, it was uniformly stirred, and 62.5 g of anhydrous ethaffin (0.694 mol) was added to the reactor, and 162.5 g of trichloride gas (1.39 mol) was slowly delivered from the bottom of the reactor (uniform and continuous access. The time is 2 h); the reaction temperature is controlled between 15 to 20 ° C during the addition of the addition; during the reaction, the resulting exhaust gas is discharged from the top of the reactor to absorb and remove hydrochloric acid having a concentration of 20% after hydrogen fluoride. 780G;
[0027](3) After t...
Embodiment 2
[0030]The preparation method of lithium boric acid acid salted acid, including the following steps:
[0031](1) in 1#In the reactor, 200 kg of fluorinated lithium fluoride (7.72 kmol) was 0.2 h at a temperature of 1540 kg of no hydrogen fluoride (77 kmol) at -20 to 0 ° C, and the hydrogen fluoride solution of lithium fluoride was obtained.
[0032](2) Put the hydrofluoric acid solution of lithium fluoride into 2#In the reactor, the stirring was mixed, 346 kg of anhydrous ethaffin (3.8 kmol) was added, and 1080 kg of a boron tap gas (9.2 kmol) was passed from the bottom of the reactor (uniform and continuous access, the entry time was 3 h); the feed process The control reaction temperature is between 5 to 15 ° C; during the reaction, the resulting gas is discharged from the top of the reactor to absorb the two-stage refinement film and remove hydrogen fluoride. 3710 kg;
[0033](3) After the endo-boron gas is processed, the mixture is continued for 30 min to complete the reaction, then the re...
Embodiment 3
[0037]The preparation method of lithium boric acid acid salted acid, including the following steps:
[0038](1) in 1#In the reactor, 1000 kg of lithium fluoride (38.6 kmol) was reactive with 3080 kg of no hydrogen fluoride (154 kmol) at 10 to 20 ° C for 2 h, and the fluoride solution of lithium fluoride was degraded.
[0039](2) Enter the fluoride solution of fluoride into 2#In the reactor, the stirring was mixed, and 1900 kg of anhydrous oxycidal acid (21.1 kmol) was added, and 6753 kg of a boron tantramid (57.6 kmol) was transferred from the bottom of the reactor (uniformly continuous access, the access time was 4 h); The control reaction temperature is between 5 and 10 ° C; during the reaction, the resulting gas is discharged from the top of the reactor to absorb and remove hydrogen hydrogen acid after hydrogen fluoride. 18450 kg;
[0040](3) After the completion of trichloride gas is formed, stirring is continued for 30 min to complete the reaction, and then the reaction liquid is filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com