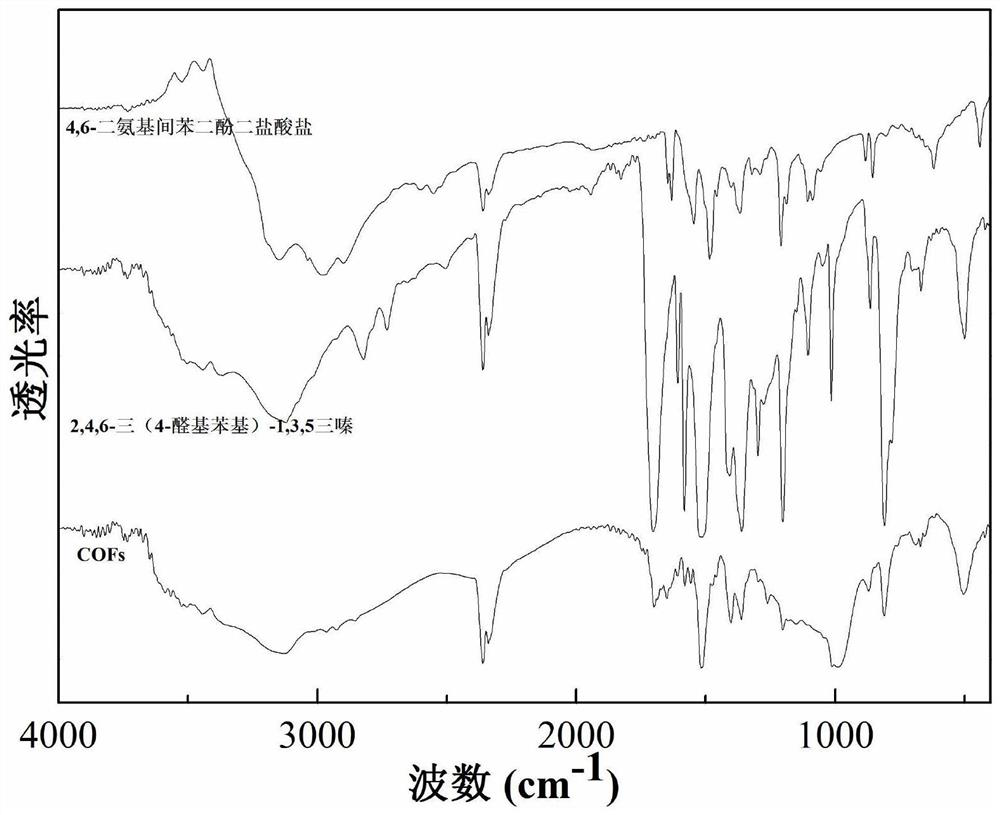
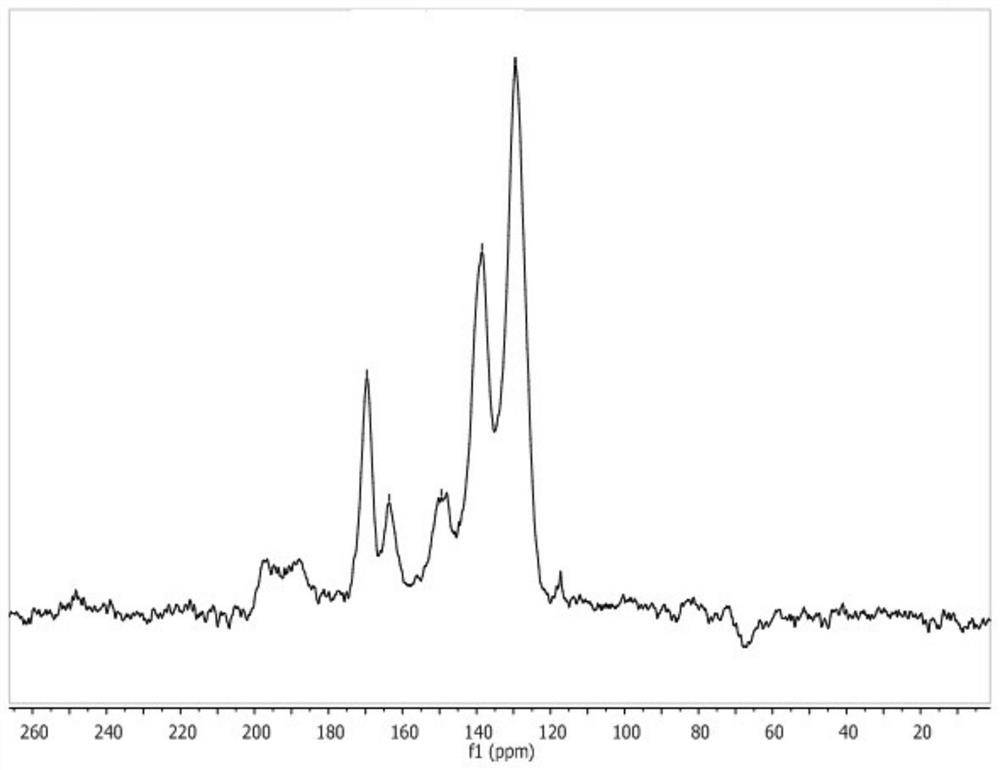
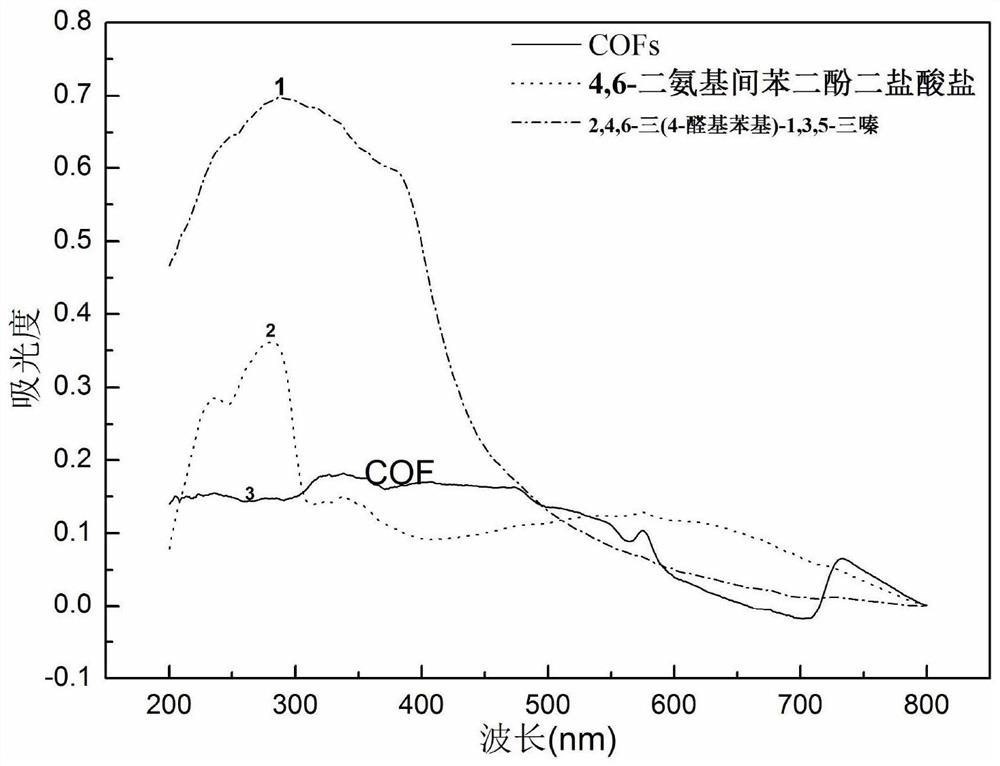
Preparation method of lamellar two-dimensional porous covalent organic framework material
A covalent organic framework and lamellar technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, inorganic chemistry, etc., can solve the problem of slow proton reduction process, low photostability and low crystallinity and other problems, to achieve the effects of high visible light catalytic hydrogen production performance, high responsiveness, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A preparation method of a lamellar two-dimensional porous covalent organic framework material of the present invention is specifically implemented according to the following steps:
[0030] Step 1: carry out dehydrochloric acid treatment on 4,6-diaminoresorcinol dihydrochloride to obtain 4,6-diaminoresorcinol; specifically:
[0031] Step 1.1: Weigh 95.8 mg of raw material 4,6-diaminoresorcinol dihydrochloride;
[0032] Step 1.2: Put the raw materials weighed in step 1.1 into a pressure tube, and add 50 mL of polyphosphoric acid;
[0033] Step 1.3: degas the pressure tube containing the raw materials and solvent in step 1.2 with nitrogen gas for three times to ensure that the reaction is carried out under anhydrous and oxygen-free conditions;
[0034] Step 1.4: Stir the pressure tube of Step 1.3 at 70°C for 8h;
[0035] Step 2: Using 4,6-diaminoresorcinol and 2,4,6-tris(4-aldophenyl)-1,3,5-triazine as raw materials to prepare COFs material;
[0036] Step 2.1: Weigh 11...
Embodiment 2
[0048] A preparation method of a lamellar two-dimensional porous covalent organic framework material of the present invention is specifically implemented according to the following steps:
[0049] In step 1, 4,6-diaminoresorcinol dihydrochloride is subjected to dehydrochloric acid treatment to obtain 4,6-diaminoresorcinol;
[0050] Specifically: 4,6-diaminoresorcinol and polyphosphoric acid were added to the pressure tube, after three nitrogen degassing treatments, and then under nitrogen protection, the reaction was carried out in an oil bath at 80 °C for 8 hours, and the removal of hydrochloric acid;
[0051] Wherein, every 9mmol of 4,6-diaminoresorcinol corresponds to 1L of polyphosphoric acid;
[0052] Step 2, add 2,4,6-tris(4-aldolphenyl)-1,3,5-triazine to 4,6-diaminoresorcinol, then carry out temperature-programmed reaction, wait for the end of the reaction after cooling to room temperature, adding distilled water to the reaction solution, suction filtration, repeatedl...
Embodiment 3
[0056] A preparation method of a lamellar two-dimensional porous covalent organic framework material of the present invention is specifically implemented according to the following steps:
[0057] In step 1, 4,6-diaminoresorcinol dihydrochloride is subjected to dehydrochloric acid treatment to obtain 4,6-diaminoresorcinol;
[0058] Specifically: 4,6-diaminoresorcinol and polyphosphoric acid were added to the pressure tube, after three nitrogen degassing treatments, and then under nitrogen protection, the reaction was carried out in an oil bath at 80 °C for 8 hours, and the removal of hydrochloric acid;
[0059] Wherein, every 10mmol of 4,6-diaminoresorcinol corresponds to 1L of polyphosphoric acid;
[0060] Step 2, add 2,4,6-tris(4-aldolphenyl)-1,3,5-triazine to 4,6-diaminoresorcinol, then carry out temperature-programmed reaction, wait for the end of the reaction after cooling to room temperature, adding distilled water to the reaction solution, suction filtration, repeated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com