Entecavir composition and preparation method thereof
A technology of entecavir and its composition, which is applied in the field of entecavir composition and its preparation, can solve the problems of material layering or compressed tablet hardness, unfavorable industrialization, uneven mixing, etc., and achieve improved content uniformity and stable dissolution rate stability, avoiding differences between single doses, avoiding the effect of uneven mixing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
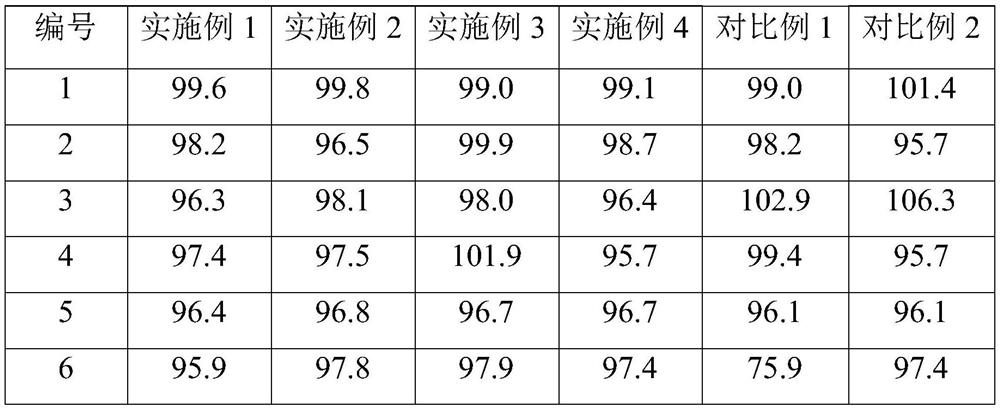
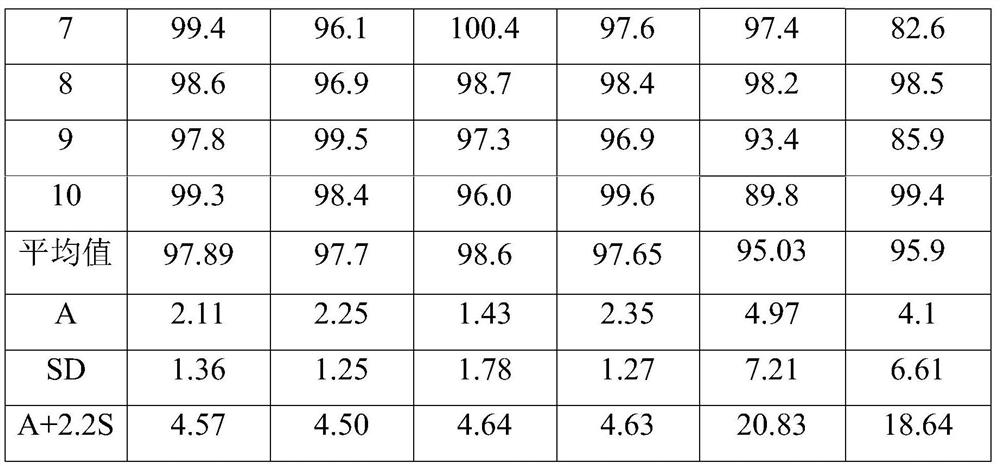
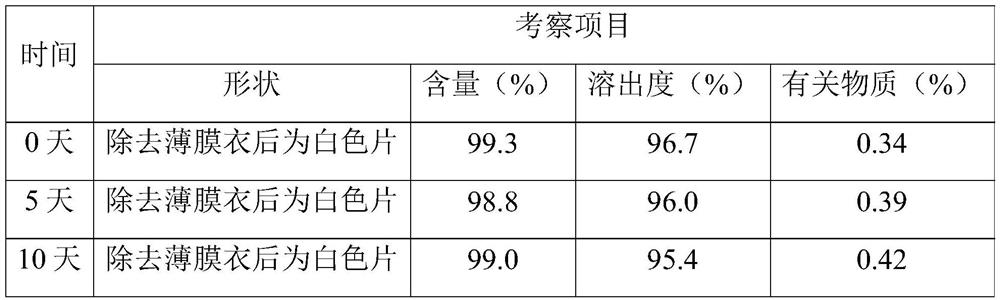
Examples
Embodiment 1
[0030] To prepare 1000 entecavir tablets of 0.5 mg specification, the specific raw materials and dosage involved are as follows:
[0031] Entecavir 0.5g
[0032] Hypromellose 5.25g
[0033] Calcium hydrogen phosphate 92.25g
[0034] Self-crosslinking povidone 9g
[0036] The preparation method comprises the following process steps:
[0037] a. Dissolve entecavir and hypromellose in 70 g of water, and heat it in a water bath to 60° C. to dissolve to obtain the main drug solution;
[0038] b. Use the single nozzle spray system on the wet granulator to atomize and spray the main drug solution onto the premix composed of calcium hydrogen phosphate, magnesium stearate and self-crosslinking povidone in the rotation, and the mist The pressure is 0.2Mpa, the rotation speed of the premix is 120r / min, and then dried in a vacuum oven at 50°C to remove moisture to obtain the entecavir composition, which is divided into 1000 parts and compressed into ...
Embodiment 2
[0040] To prepare 1000 entecavir tablets of 0.5 mg specification, the specific raw materials and dosage involved are as follows:
[0041] Entecavir 0.5g
[0042] Povidone 1g
[0043] Microcrystalline cellulose 73g
[0044] Croscarmellose Sodium 8.3g
[0045] Magnesium stearate 0.8g.
[0046] The preparation method comprises the following process steps:
[0047] a. Dissolve entecavir and povidone in 75g of water, and heat to 70°C in a water bath to dissolve to obtain the main drug solution;
[0048] b. Use the single nozzle spray system on the wet granulator to atomize and spray the main drug solution to the premix composed of microcrystalline cellulose, magnesium stearate and croscarmellose sodium in the rotation Above, the atomization pressure is 0.3Mpa, the rotation speed of the premix is 200r / min, and then dried in a vacuum oven at 55°C to remove moisture to obtain the entecavir composition, which is divided into 1000 parts and compressed into tablets to obtain 1000 ...
Embodiment 3
[0050]To prepare 1000 entecavir tablets of 1 mg specification, the specific raw materials and dosage involved are as follows:
[0051] Entecavir 1g
[0052] Hydroxypropyl cellulose 10.5g
[0053] Lactose 184.5g
[0054] Low-substituted hydroxypropyl cellulose 20g
[0055] Magnesium stearate 2g.
[0056] The preparation method comprises the following process steps:
[0057] a. Dissolve entecavir and hypromellose in 160 g of water, and heat it in a water bath to 80° C. to dissolve to obtain the main drug solution;
[0058] b. Use the single nozzle spray system on the wet granulator to atomize and spray the main drug solution onto the premix composed of calcium hydrogen phosphate, magnesium stearate and self-crosslinking povidone in the rotation, and the mist The pressure is 0.4Mpa, the rotation speed of the premix is 50r / min, and then dried in a vacuum oven at 60°C to remove moisture to obtain the entecavir composition, which is divided into 1000 parts and compressed into...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com