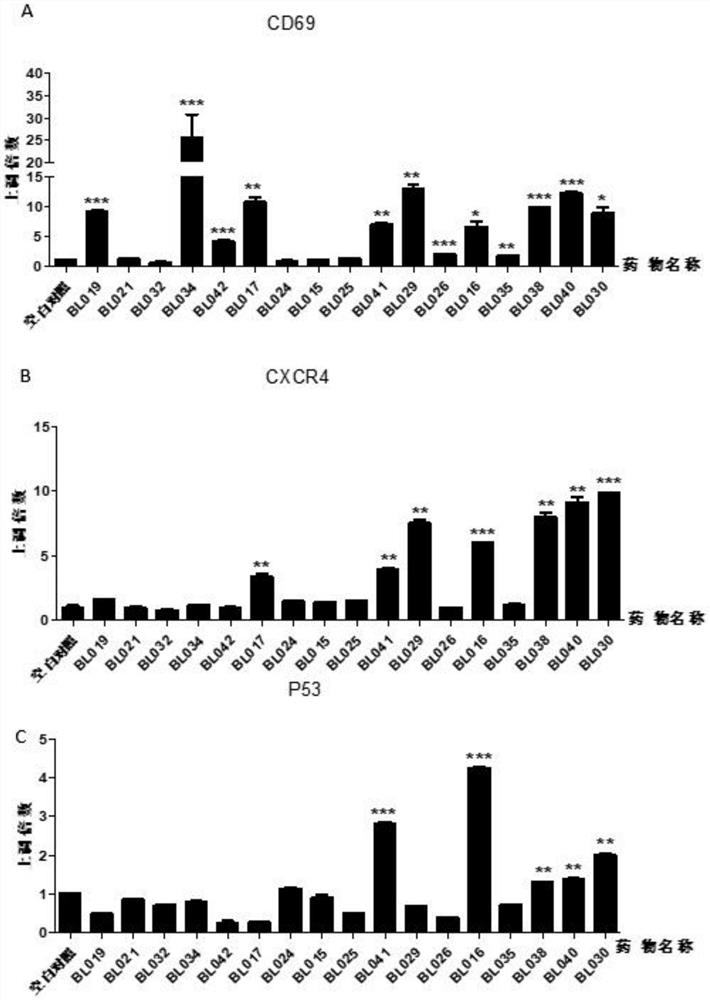
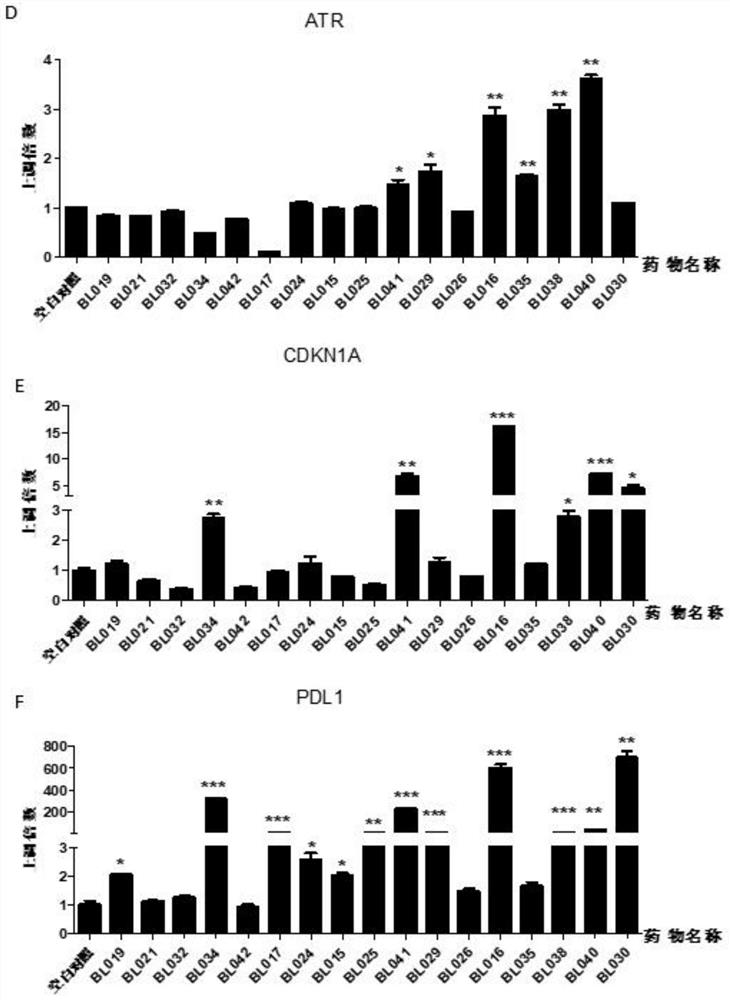
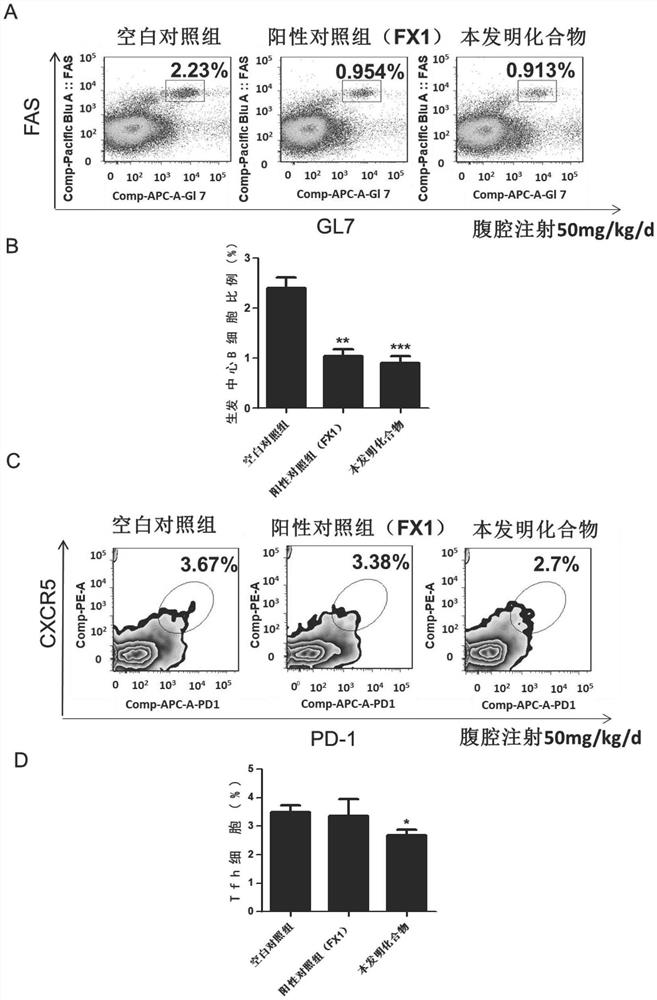
BCL6-targeting aromatic ring five-membered aromatic heterocyclic micromolecular organic compound and derivative and application thereof
A technology of organic compounds and aromatic heterocycles, applied in the field of small molecule organic compounds with aromatic rings and five-membered aromatic heterocycles, can solve the problems of inability to up-regulate BCL6, low cell proliferation inhibitory activity, and no anti-tumor activity, etc., to achieve Significant anti-tumor activity, inhibition of proliferation, and inhibition of germinal center formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0171]The purity results of the compounds obtained in the experiment were all obtained from an Agilent 1200series LC system high-performance liquid chromatography analyzer (chromatographic conditions: Zorbax XDB-C18 (4.6×150nm, 5μm), column 40°C, mobile phase MeOH / H 2 0, the operating flow rate is 1.5mL / min, the ultraviolet detection wavelength is 254nm, and the injection volume is 10 μ L); the nuclear magnetic resonance spectrometer is Bruker 500 or Bruker 600 type (internal standard: TMS, and the use solvent is DMSO-d 6 ); the purification of the reaction intermediate and the final product all use a chromatographic column (200-300 mesh of silica gel used), and the silica gel used is purchased from Qingdao Ocean Chemical Factory. All solvents were re-distilled before use, and the anhydrous solvents used were obtained by drying according to standard methods; unless otherwise specified, all reactions were carried out under nitrogen protection and the progress of the reaction was...
Embodiment 1-2
[0186] Example 1-2: (2-((6-((5-((3S,5R)-3,5-dimethylpiperazinyl)-[1,2,4]triazolo[1,5 -a] Preparation of pyrimidin-7-yl)amino)-1-methyl-2-oxo-1,2-dihydroquinolin-3-yl)oxy)acetyl)alanine (BL008).
[0187] Dissolve 3-hydroxy-1-methyl-6-nitroquinolin-2(1H)-one (713mg, 3.24mmol) and methyl 2-bromoacetate (991mg, 6.48mmol) in DMF (10mL), Cesium carbonate (3.17 g, 9.72 mmol) was added to the reaction liquid, and stirred overnight at room temperature. After the completion of the reaction, conventional processing, column chromatography to obtain 2-((1-methyl-6-nitro-2-oxo-1,2-dihydroquinolin-3-yl) oxy)methyl acetate ( 833mg, 88%)
[0188] Dissolve methyl 2-((1-methyl-6-nitro-2-oxo-1,2-dihydroquinolin-3-yl)oxy)acetate (833 mg, 2.85 mmol) in 30 mL of methanol in a mixed solvent with water (MeOH:H 2 O=4:1), LiOH (718 mg, 17.10 mmol) was added, and stirred overnight at room temperature. After the reaction, methanol was removed, then dilute hydrochloric acid was added dropwise to adjus...
Embodiment 1-3
[0193] Example 1-3: 2-((6-((5-((3S,5R)-3,5-dimethylpiperazinyl)-[1,2,4]triazolo[1,5- a] Preparation of pyrimidin-7-yl)amino)-1-methyl-2-oxo-1,2-dihydroquinolin-3-yl)oxy)-N-phenylacetamide (BL009)
[0194] Take 2-((6-((5-((3S,5R)-3,5-dimethylpiperazinyl)-[1,2,4]triazolo[1,5-a]pyrimidine-7 -yl)amino)-1-methyl-2-oxo-1,2-dihydroquinolin-3-yl)oxy)methyl acetate (BL007) (323mg, 0.66mmol) and LiOH (138mg, 3.27 mmol), dissolved in 30mL of mixed solvent of methanol and water (MeOH:H 2 O=4:1) and stirred overnight at room temperature. After the reaction, methanol was removed, and dilute hydrochloric acid was added dropwise to adjust the acidity until the solid was no longer precipitated, and the obtained solid 2-((6-((5-((3S,5R)-3,5-dimethylpiperene Azinyl)-[1,2,4]triazolo[1,5-a]pyrimidin-7-yl)amino)-1-methyl-2-oxo-1,2-dihydroquinoline-3 -yl)oxy)acetic acid (314 mg, 99%).
[0195] Take 2-((6-((5-((3S,5R)-3,5-dimethylpiperazinyl)-[1,2,4]triazolo[1,5-a]pyrimidine-7 -yl)amino)-1-meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com