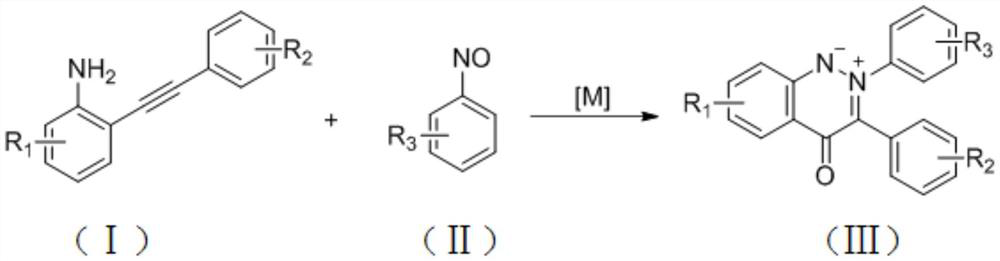
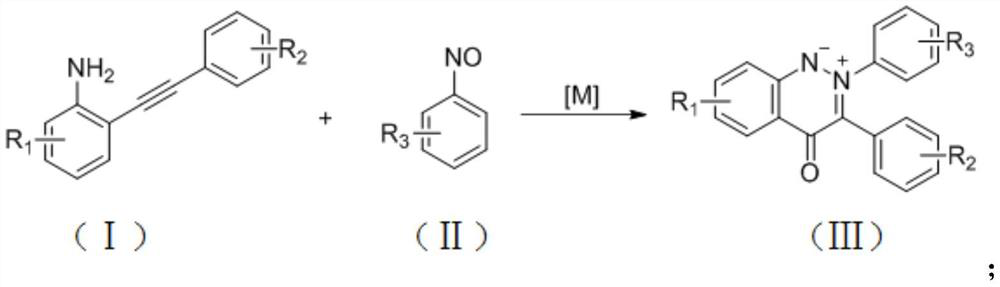
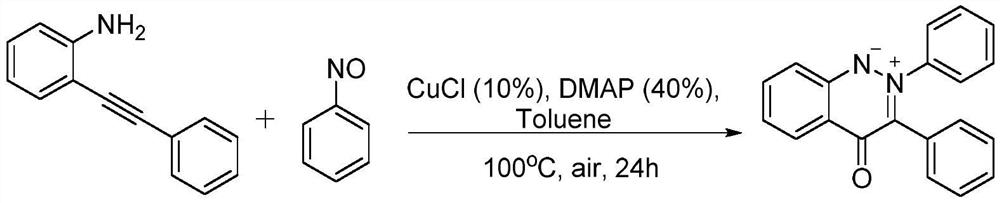
Synthesis method for cinnoline salt compound
A technology of cinnoline salt compound and synthesis method, which is applied in the field of synthesis of cinnoline salt compound, can solve problems such as reduced practicality, and achieve the effects of good product yield, good research value and application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: 2,3-Diphenyl-4-oxocinnoline ylide
[0023]
[0024] 2-Phenylethynylaniline (96.6 mg, 0.5 mmol), nitrosobenzene (80.3 mg, 0.75 mmol), CuCl (5.0 mg, 0.05 mmol), 4-dimethylaminopyridine ( 24.4mg, 0.2mmol), toluene (1.5mL), and then seal the tube. Put the reaction mixture into an oil bath at 100°C and stir for 24 hours, take it out and cool it to room temperature, transfer the original reaction solution, add an appropriate amount of ethyl acetate to wash, mix the original solution with the washing solution, concentrate under reduced pressure and adsorb on silica gel, and then directly carry out Silica gel chromatographic separation, using a mixture of dichloromethane and ethyl acetate at a volume ratio of 50:1 as the eluent, was obtained after column chromatography with a yield of 74%. 1 H-NMR (500MHz, DMSO): δ8.25(d, J=8.0Hz, 1H), 7.95(d, J=8.5Hz, 1H), 7.88(t, J=6.5Hz, 1H), 7.65(t ,J=7.0Hz,1H),7.58-7.57(m,2H),7.40(t,J=3.5Hz,3H),7.35(d,J=7.5Hz,2H),7.30-7.26(...
Embodiment 2
[0025] Example 2: 2-phenyl-3-(4-(methoxycarbonyl)phenyl)-4-oxocinnoline ylide
[0026]
[0027] Add 4-((2-aminophenyl)ethynyl)methyl benzoate (125.6mg, 0.5mmol), nitrosobenzene (80.3mg, 0.75mmol), CuCl (5.0mg, 0.05mmol), 4-dimethylaminopyridine (24.4mg, 0.2mmol), toluene (1.5mL), and then seal the tube. Put the reaction mixture in an oil bath at 100°C and stir for 24 hours, take it out and cool it to room temperature, transfer the original reaction solution, add an appropriate amount of ethyl acetate to wash, mix the original solution and washing solution, concentrate under reduced pressure and adsorb on silica gel, and carry out directly Silica gel chromatographic separation, using a mixture of dichloromethane and ethyl acetate at a volume ratio of 50:1 as the eluent, obtained after column chromatography with a yield of 86%. 1 H-NMR (500MHz, CDCl 3 ): δ8.42(d, J=8.0Hz, 1H), 7.91(d, J=8.0Hz, 3H), 7.76(t, J=8.0Hz, 1H), 7.58(t, J=7.5Hz, 1H ),7.35(d,J=9.5Hz,7H),3.87(s,3H). ...
Embodiment 3
[0028] Example 3: 2,3-diphenyl-4-oxo-7-chlorocinnoline ylide
[0029]
[0030] 5-Chloro-2-(phenylethynyl)aniline (113.8mg, 0.5mmol), nitrosobenzene (80.3mg, 0.75mmol), CuCl (5.0mg, 0.05mmol), 4 - Dimethylaminopyridine (24.4 mg, 0.2 mmol), toluene (1.5 mL). Then, seal the tube. Put the reaction mixture in an oil bath at 100°C and stir for 24 hours, take it out and cool it to room temperature, transfer the original reaction solution, add an appropriate amount of ethyl acetate to wash, mix the original solution and washing solution, concentrate under reduced pressure and adsorb on silica gel, and carry out directly Silica gel chromatographic separation, using a mixture of dichloromethane and ethyl acetate at a volume ratio of 50:1 as the eluent, was obtained after column chromatography with a yield of 73%. 1 H-NMR (500MHz, CDCl 3 ): δ8.18(d, J=9.0Hz, 1H), 7.76(d, J=1.5Hz, 1H), 7.36(dd, J=8.5Hz, J=2.0Hz, 1H), 7.27-7.24(m ,5H),7.18-7.15(m,5H). 13 C-NMR (500MHz, CDCl 3 ): δ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com