Method for constructing infected animal model by using coxsackie A2 virus strain and application of infected animal model
A technology for infecting animals and virus strains, applied in the field of biomedicine, can solve the problem of lack of animal models of CVA2 infection, and achieve the effect of preventing and controlling hand, foot and mouth disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Coxsackie A2 virus strain constructs the method for animal model:
[0048] 1) Cultivation of CVA2 virus strain
[0049] 1.1) Acquisition of CVA2 virus strain HN202009
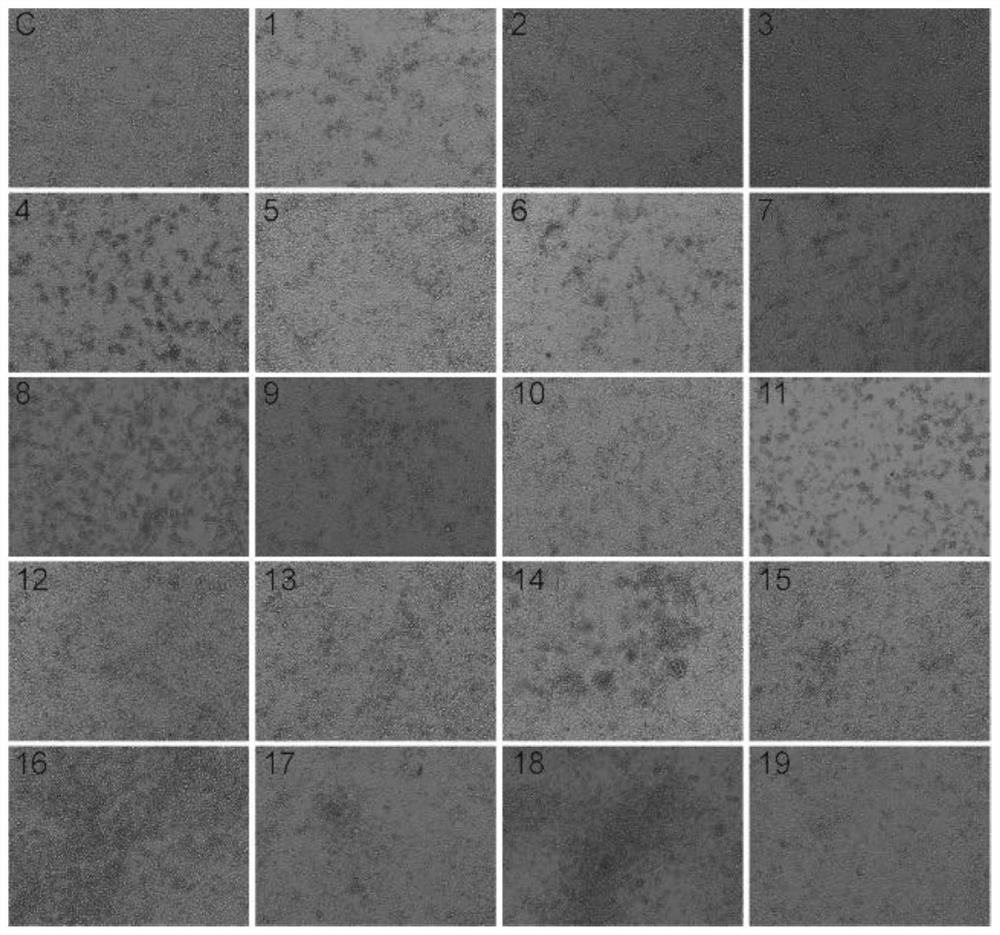
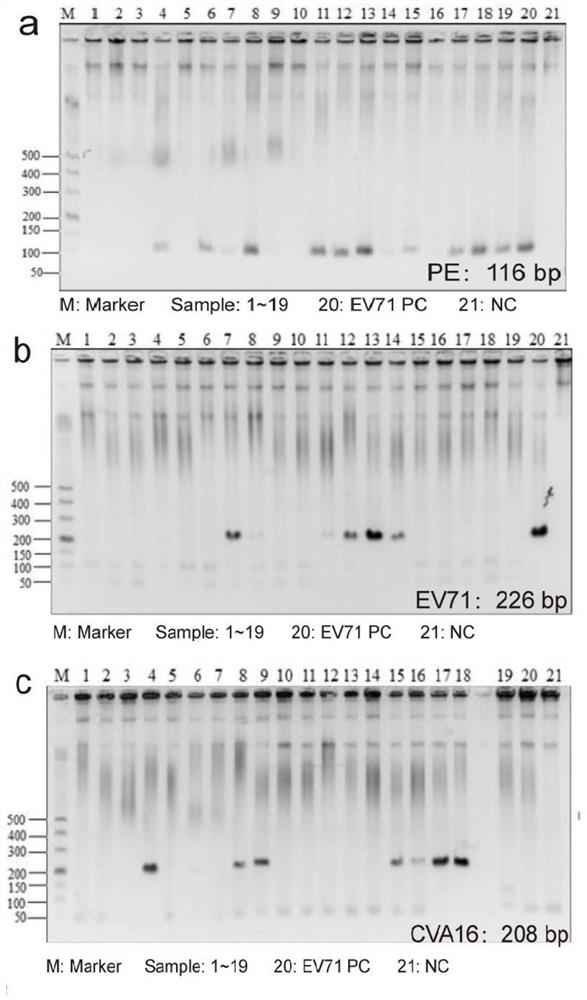
[0050] Clinical diagnosis of HFMD cases was made according to the diagnostic criteria of the Ministry of Health, and the feces of 19 children who had symptoms of vesicular rash on hands, feet, oral mucosa or buttocks and were diagnosed with HFMD were collected from January 2017 to December 2017 sample.
[0051] Identification of CVA2 strains from clinical isolates of patients with hand, foot and mouth disease. A total of 19 patients diagnosed with HFMD were included in this study, and their clinical characteristics are shown in Table 1. Among them, 8 cases (accounting for 42.11%) showed enterovirus (PE) infection, 3 cases (accounting for 15.79%) were positive for EV71 immunoglobulin M (IgM), and 5 cases (accounting for 26.32%) were positive for C-reactive protein (CRP) was positive. Stool samples fr...
experiment example 1
[0088] Histopathological and immunohistochemical analysis:
[0089] 1.1 Histopathological examination
[0090] After finally determining to set up the optimal combination condition of CVA2 infection model, according to the optimization condition of embodiment 1 (muscle injection inoculation, dosage is 10 4 TCID 50 / only, 5-day-old neonatal BALB / c mice) to establish a mouse CVA2 infection model, and 5-day-old neonatal BALB / c mice intramuscularly injected with the same amount of maintenance solution as the control group. In order to identify the histopathological characteristics of CVA2-infected mice, the present invention euthanized the infected mice and control mice after the mice were infected with CVA2 7dpi, and obtained brain (brain), lung (lung), skeletal muscle (skeletal muscle) , spinal cord and heart samples. A portion of the above sample was fixed in 10% paraformaldehyde for 48 hours. After fixation, paraffin-embedded organs and tissues were sectioned into 5 μm sec...
experiment example 2
[0100] Evaluation of animal models:
[0101] 2.1 CVA2 whole virus vaccine, preparation of antiviral serum and determination of anti-CVA2 antibody titer
[0102] By combining 37% formaldehyde (GB / T685-1993) with 2.45×10 7 TCID 50 / mL CVA2 strain HN202009 was mixed, and the inactivated CVA2 suspension was prepared according to the ratio of formaldehyde concentration of 1:4,000 (vol / vol). The virus suspension was then incubated at 37°C for 3 days. After 3 weeks of continuous RD cell culture and blind passage, RD cells inoculated with inactivated CVA2 did not develop cytopathic changes, and the virus was confirmed to be completely inactivated. Combine the inactivated virus suspension with inject TM Equal volumes of alum adjuvant (Thermo) were mixed to obtain a mixed solution, which was the CVA2 whole virus vaccine.
[0103]Inoculate 200 μL of inactivated CVA2 emulsion into six-week-old adult female BALB / c mice by intraperitoneal injection, and immunize adult female mice twice...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com