Bilastine composition and novel preparation process thereof
A technology of bilastine and bilastine tablets, which is applied in the field of pharmaceutical preparations, can solve problems affecting product quality, loose tablets and splits, and difficult to reach, and achieve the effects of improving sticking phenomenon, improving requirements, and stabilizing quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
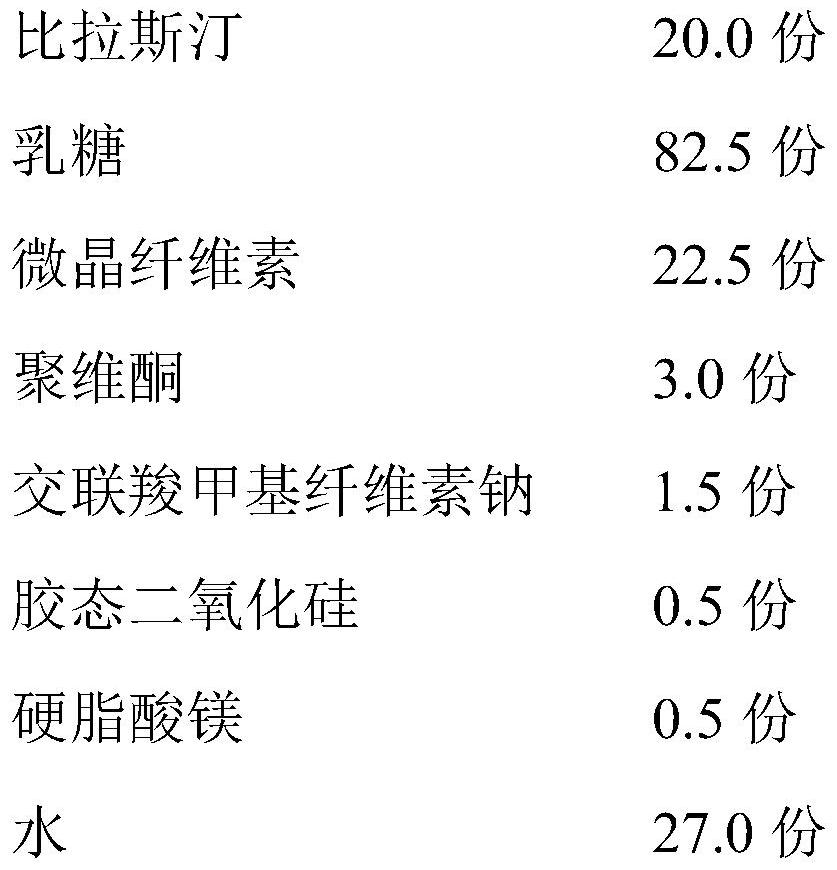
[0029] 1. Preparation prescription
[0030]
[0031] 2. Preparation process
[0032] 1) After mixing bilastine, 62.5 parts of lactose, microcrystalline cellulose and croscarmellose sodium in the formula quantity, pass through a 60-mesh sieve, and dissolve povidone in water to prepare a solution; (environment Humidity: 55%±10%)
[0033] 2) Fluidized bed granulation parameters are: material temperature: 35-55°C; air inlet temperature: 40-90°C; air volume: 7-35m 3 / h, the spray speed of the adhesive is 1-5rpm, and it starts to dry after the adhesive solution is sprayed;
[0034] 3) Fluidized bed drying parameters are: material temperature: 30-60°C; air inlet temperature: 35-95°C; air volume: 5-40m 3 / h, when the temperature of the material reaches 60°C, the material is discharged;
[0035] 4) granulate the dried particles through a 40-mesh sieve;
[0036] 5) Add colloidal silicon dioxide and remaining lactose to the sized granules, and mix evenly; add magnesium stearate, ...
Embodiment 2
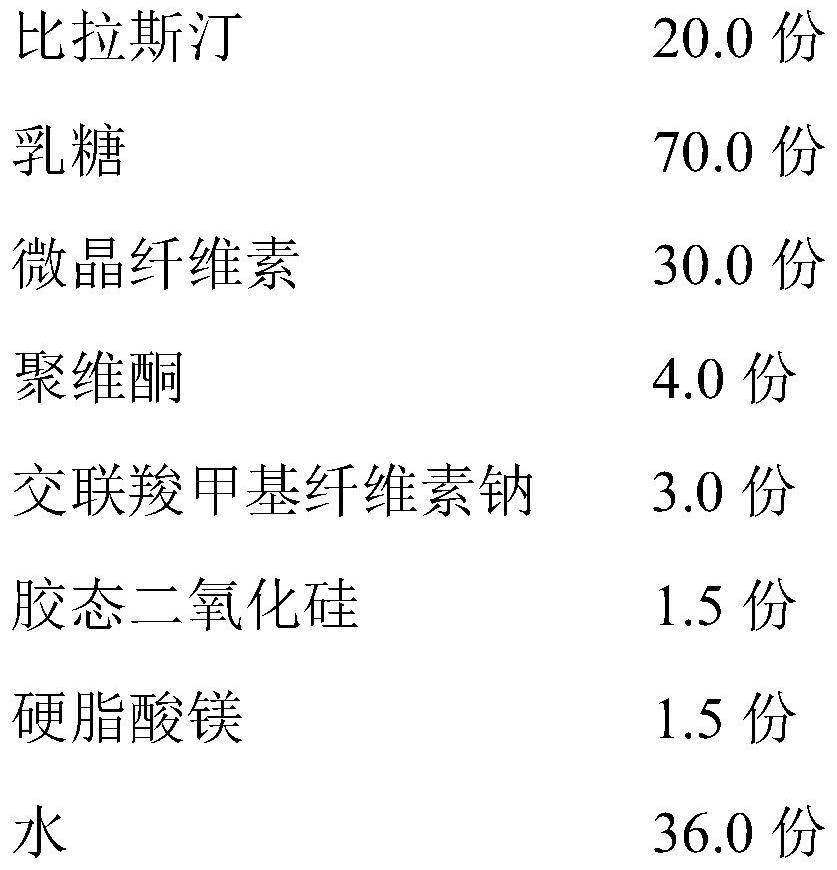
[0038] 1. Preparation prescription
[0039]
[0040] 2. Preparation process
[0041] 1) After mixing the amount of bilastine, 35 parts of lactose, microcrystalline cellulose and croscarmellose sodium, pass through an 80-mesh sieve, and dissolve povidone in water to prepare a solution; (environmental humidity : 55%±10%)
[0042] 2) Fluidized bed granulation parameters are: material temperature: 35-55°C; air inlet temperature: 40-95°C; air volume: 7-35m 3 / h, the spray speed of the adhesive is 1-5rpm, and it starts to dry after the adhesive solution is sprayed;
[0043] 3) Fluidized bed drying parameters are: material temperature: 30-60°C; inlet air temperature: 35-95°C; air volume: 5-42m 3 / h, when the temperature of the material reaches 60°C, the material is discharged;
[0044] 4) granulate the dried particles through a 40-mesh sieve;
[0045] 5) Add colloidal silicon dioxide and remaining lactose to the sized granules, and mix evenly; add magnesium stearate, mix for ...
Embodiment 3
[0047] 1. Preparation prescription
[0048]
[0049]
[0050] 2. Preparation process
[0051] 1) After mixing the formula amount of bilastine, 30 parts of lactose, microcrystalline cellulose and croscarmellose sodium, pass through a 100-mesh sieve, and dissolve povidone in water to prepare a solution; (environmental humidity : 55%±10%)
[0052] 2) Fluidized bed granulation parameters are: material temperature: 35-55°C; air inlet temperature: 40-95°C; air volume: 7-35m 3 / h, the spraying speed of the adhesive is 1-4rpm, and it starts to dry after the adhesive solution is sprayed;
[0053] 3) Fluidized bed drying parameters are: material temperature: 30-60°C; inlet air temperature: 35-95°C; air volume: 5-45m 3 / h, when the temperature of the material reaches 60°C, the material is discharged;
[0054] 4) the dried particles are sized through a 24-mesh sieve;
[0055] 5) Add colloidal silicon dioxide and remaining lactose to the sized granules, and mix evenly; add magnes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com