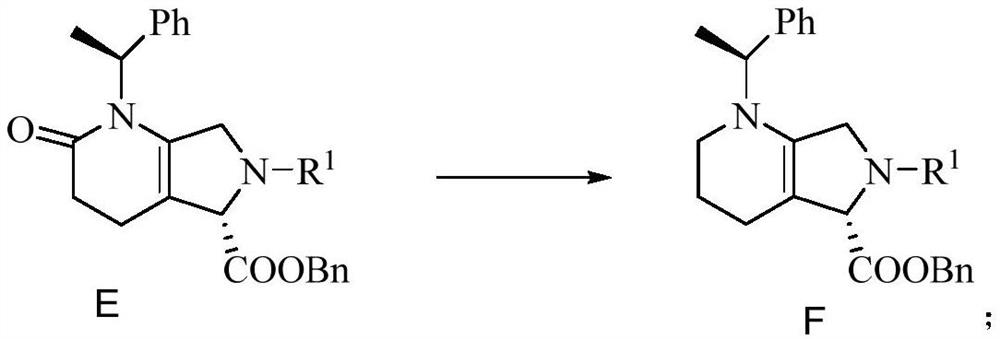
Preparation method of intermediate
A compound and reaction technology, applied in the field of preparation of intermediates, can solve the problems of difficulty in obtaining a single configuration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Embodiment 1 prepares compound C
[0078] Add 20.0g of L-hydroxyproline, 32.0g of sodium bicarbonate and 300.0mL of water into a 500mL one-necked flask, and stir evenly at room temperature; add dropwise 60.0mL of toluene solution containing 30.0g of benzyl chloroformate, after the addition is complete, stir at room temperature for 16h . Stop the reaction, let the layers rest, separate the organic phase, wash the aqueous phase twice with 50mL ether, adjust the pH of the system to 2 with concentrated hydrochloric acid, extract three times with 250mLEA, combine the organic phases, dry with anhydrous sodium sulfate, and filter Concentration under reduced pressure gave 37.3 g of oily compound A, HPLC content: 97.8%; yield 90.2%.
[0079] 7.82g of Compound A (HPLC content: 97.8%) was dissolved in 420mL of acetone, 12mL of prepared 2mol / L Jones reagent was added dropwise, stirred at room temperature for 0.5h, after the reaction was completed, concentrated to dryness under red...
Embodiment 2
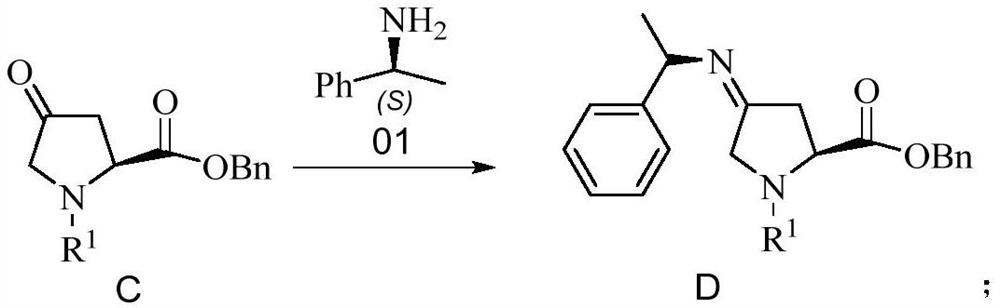
[0081] Embodiment 2 prepares compound D
[0082]
[0083] 3.83g of compound C (HPLC content: 98.4%) was dissolved in 100mL of toluene, and 1.76g of S-methylbenzylamine was added dropwise. After the dropwise addition, the temperature was raised to 110°C, and kept stirring for 18h. After cooling to room temperature, the reaction system was concentrated under reduced pressure to obtain an oil compound D: 4.89 g, HPLC content: 96.7%, yield 96.9%.
[0084] LC-MS: [M+1]=457.1; 1 H-NMR(400MHZ):δ7.37-7.23(m,15H),5.31(s,2H),5.13(s,2H),4.43-4.53(m,1H),3.52-3.69(m,5H), 1.32(m,3H).
Embodiment 3
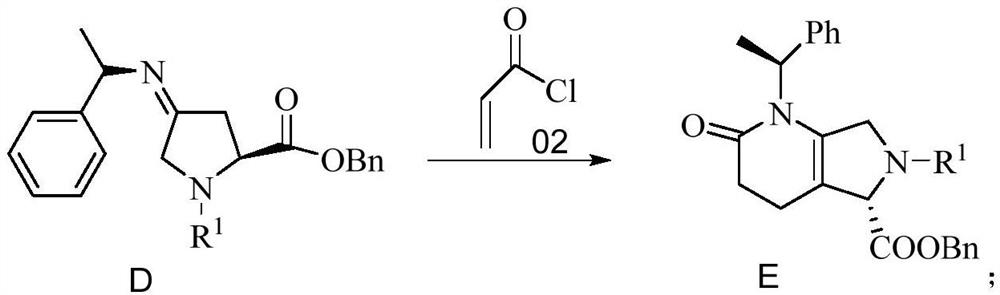
[0085] Embodiment 3 prepares compound E
[0086]
[0087] 3.97g of compound D (HPLC content: 96.7%) was dissolved in 40mL of THF, 7.50g of pyridine was added, and after stirring at room temperature for 30min, 2.26g of acryloyl chloride was added dropwise. After the dropwise addition, the system was heated to 70°C and kept stirring 2h. The reaction system was concentrated to dryness under reduced pressure, then dissolved in 100 mL of ethyl acetate, washed with 1 mol / L dilute hydrochloric acid (100 mL×4), dried the organic phase, filtered, and the filtrate was concentrated to give an oil compound E: 4.02 g, HPLC content : 96.3%; Yield: 90.2%.
[0088] LC-MS: [M+1]=511.2; 1 H-NMR (400MHZ): δ7.39-7.21(m,15H),5.3(s,2H),5.1(s,2H),4.63(s,1H),4.53-4.48(m,1H),3.69( s,2H), 2.34-2.23(m,4H), 1.31(m,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com