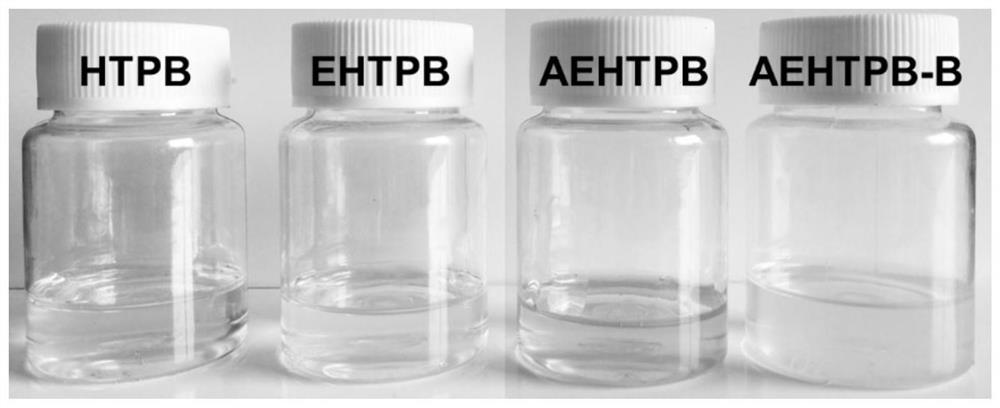
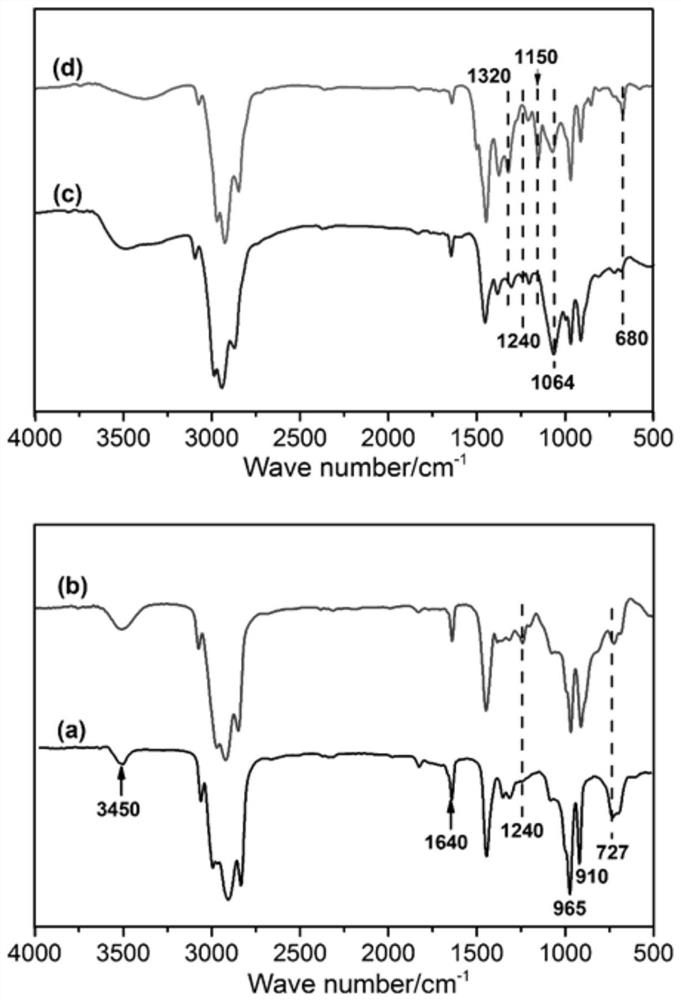
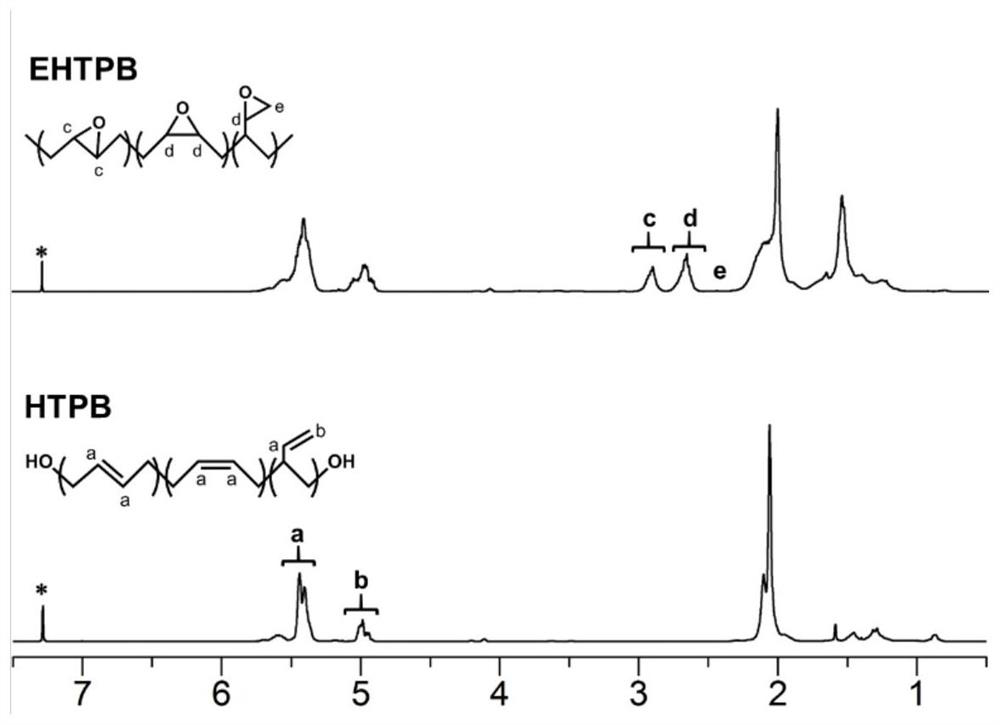
A modified hydroxyl-terminated polybutadiene and its preparation method and application
A modified technology of hydroxyl-terminated polybutadiene, which is applied in the production of offensive equipment, explosives, compressed gas, etc., can solve the problem of weak bonding between the adhesive matrix and the filler interface, single functionality, and complex propellant formulations, etc. problems, to achieve the effect of improving thermodynamic compatibility, improving mechanical properties, and accelerating solid-liquid wetting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] In the present embodiment, the molecular structure of multifunctional modified hydroxyl-terminated polybutadiene (AEHTPB-B) containing ring boronic acid ester group is as follows:
[0053]
[0054] Wherein, x=44-64, y=2-5, z=3-10.
[0055] The preparation method of the AEHTPB-B of the present embodiment comprises the following specific steps:
[0056] S1. Add 30g of HTPB, 50g of toluene, 15g of glacial acetic acid, and 3g of cationic resin into the reaction vessel, stir evenly and heat up to 60°C; add 75g of H 2 o 2 , and then continue the constant temperature reaction for 8 hours; after the reaction is over, let it stand for stratification, wash the organic layer with distilled water until it is neutral, and at the same time, the washing solution will no longer discolor the starch potassium iodide test paper; A colorless and transparent viscous liquid was obtained, namely EHTPB; the epoxy value of the product was 0.35mol / 100g, and the yield was 97.8%;
[0057] S2...
Embodiment 2
[0060] The AEHTPB-B molecular structure of the present embodiment is as follows:
[0061]
[0062] Among them, x=44-64, y=2-4, z=3-6.
[0063] The method for preparing the AEHTPB-B of the present embodiment may further comprise the steps:
[0064] S1. Add 30g HTPB, 30g toluene, 30g glacial acetic acid, 1g ammonium tungstate hydrate into the reaction vessel, stir evenly and raise the temperature to 55°C, add 60g H 2 o 2, and then continue the constant temperature reaction for 6 hours; after the reaction is over, let it stand for stratification, wash the organic layer with distilled water until it is neutral, and at the same time, the washing solution no longer discolors the starch potassium iodide test paper; Transparent viscous liquid, epoxidized hydroxyl-terminated polybutadiene (EHTPB). The epoxy value of the product is 0.25mol / 100g, and the yield is 97.9%;
[0065] S2. Take 20g of the EHTPB prepared in step S1 and add it to the reaction kettle, dissolve it with 30g o...
Embodiment 3
[0068] AEHTPB-CN molecular structure is as follows in the present embodiment:
[0069]
[0070] Among them, x=44-58, y=3-5, z=8-10.
[0071] The preparation method of AETHPB-CN in this embodiment comprises the following specific steps:
[0072] S1. Add 30g of HTPB, 30g of toluene, 16g of glacial acetic acid, and 2g of cation exchange resin into the reaction vessel, stir evenly and heat up to 55°C, and dropwise add 75g of H 2 o 2 , and then continue the constant temperature reaction for 7 hours; after the reaction is over, let it stand for stratification, wash the organic layer with distilled water until it is neutral, and at the same time, the washing solution no longer discolors the starch potassium iodide test paper; the organic phase is rotary evaporated under reduced pressure at 90°C to obtain a colorless Transparent viscous liquid, namely EHTPB; the epoxy value of the product is 0.44 mol / 100g, and the yield is 97.1%;
[0073] S2. Take 20 g of the EHTPB prepared in s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com