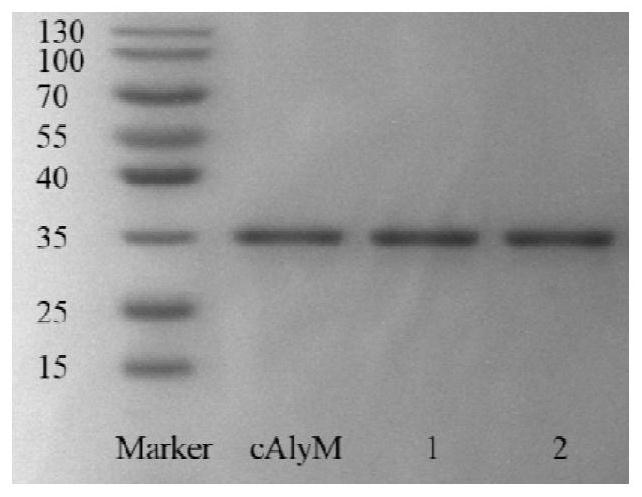
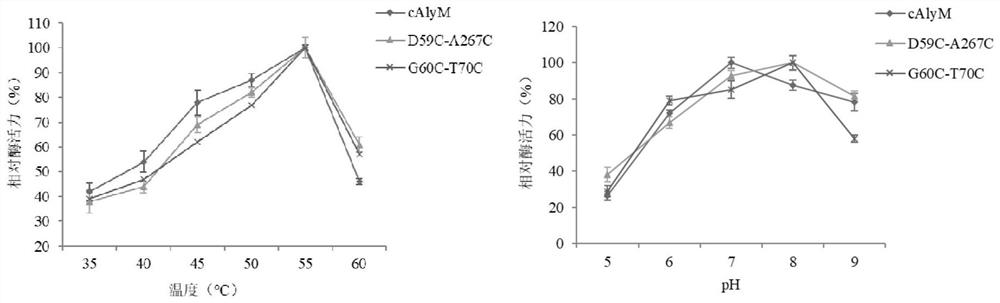
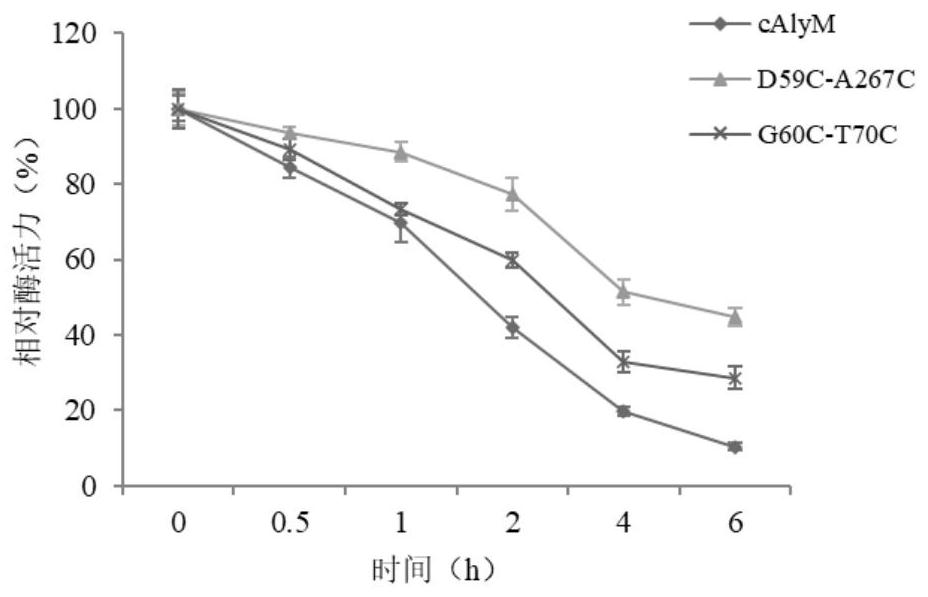
A thermal stability and high enzyme activity recombinant alginate lyase and its application
A technology of alginate lyase and algin oligosaccharides, which is applied in the field of alginate lyase, can solve the problems of unsatisfactory enzyme activity, low yield of alginate lyase, and improved thermal stability, so as to improve immunity, shorten enzyme Effect of solution time, high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Construction of mutants and their expression in Escherichia coli
[0026] In order to improve the thermal stability and activity of alginate lyase, the applicant modified the original alginate lyase with the following amino acid sequence:
[0027] MKVSCAVVLSACIASANADNNGDGKADSIKENDLNAGYADGTYFYTAADGGMVFRCPIDGYKTSTNTSYTRTELREMLRRGDTSIATQGVNGNNWVFGSAPASAREAAGGVDGVLRATLAVNHVTTTGDSGQVGRVIVGQIHANNDEPLRLYYRKLPGHSKGSVYIAHEPNGGSDSWYDMIGSRSSSASDPSDGIALDEVWSYEVKVVGNTLTVTIFRAGKDDVVQVVDMGNSGYDVADQYQYFKAGVYNQNNTGNASDYVQVTFYALEQSHD(SEQ ID NO:1)
[0028] The original alginate lyase, the nucleotide sequence of its coding gene is as follows:
[0029] ATGAAAGTAAGTTGCGCTGTCGTACTGTCTGCTTGTATTGCCAGTGCCAACGCAGACAACAATGGCGATGGCAAGGCCGACTCCATCAAGGAAAATGACCTGAATGCAGGCTATGCAGATGGCACCTACTTCTATACTGCTGCCGATGGCGGCATGGTGTTCCGCTGCCCGATCGATGGCTATAAAACATCGACCAACACGTCCTATACCCGCACCGAGCTGCGCGAGATGCTACGTCGTGGCGACACCAGCATTGCCACCCAGGGGGTCAATGGAAACAACTGGGTATTCGGCTCCGCACCCGCTTCGGCACGTGAAGCAGCCGGCGGTGTC...
Embodiment 2
[0054] Example 2: Expression of recombinant enzymes in Pichia pastoris
[0055] Using the plasmids HTa-cAlyM and HTa-D59C-A267C as templates, design primers to amplify the genes of the original enzyme and the modified enzyme. The primers are as follows:
[0056] EcoR Ⅰ-F: 5’-agagaggctgaagctgaattcATGAAAGTAAGTTGCGCTGTC-3’
[0057] Not Ⅰ-R: 5’-tgttctagaaagctggcggccgcTTAATCGTGCGACTGCTCCAG-3’
[0058] The amplification system is as follows:
[0059]
[0060]
[0061] The amplification conditions are as follows:
[0062]
[0063] After PCR amplification, use agarose gel electrophoresis at a concentration of 1% to verify whether the size and length of the PCR product bands are consistent with the target length, and then purify the PCR product with a PCR product purification kit. The pPICZαA empty plasmid stored in the laboratory was double digested with EcoRI and NotI, incubated in a water bath at 37°C for 1-3h, and inactivated at 80°C for 20min. The enzyme digestion sys...
Embodiment 3
[0077] Example 3: Preparation of Alginate Oligosaccharides
[0078] Dissolve alginate in water with pH=7.5 adjusted by NaOH, prepare 200mL alginate solution with a concentration of 6%, add 0.6mL or 0.8mL of recombinant alginate lyase D59C-A267C or cAlyM, and stir in a warm bath at 50°C for enzymolysis After 1.5h, add 0.2mL or 0.6mL of recombinant alginate lyase D59C-A267C or cAlyM, and continue enzymolysis at 50°C for 1h with stirring. The enzymolysis solution was centrifuged at 8000rpm for 10min to remove the residue, and the supernatant was the enzymolysis product.
[0079] The hydrolyzate of alginate lyase D59C-A267C was subjected to 4-fold alcohol precipitation to obtain the target product, and the crude product of algin oligosaccharide was obtained after the target product was rotary evaporated and freeze-dried, with a yield of 84.3%; oligosaccharides were determined by ESI-MS Polymerization degree and ESI-MS spectrum showed that the final products of enzymatic hydrolysi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com