A kind of preparation method of nintedanib intermediate
A technology for nintedanib and intermediates, which is applied in the field of preparation of nintedanib intermediates, can solve the problems of human harm, low purity, and reduced yield, so as to reduce harm and ensure drug quality and yield. improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of 1-acetyl-3-(ethoxy(phenyl)methylene)-2-oxoindoline-6-carboxylic acid methyl ester
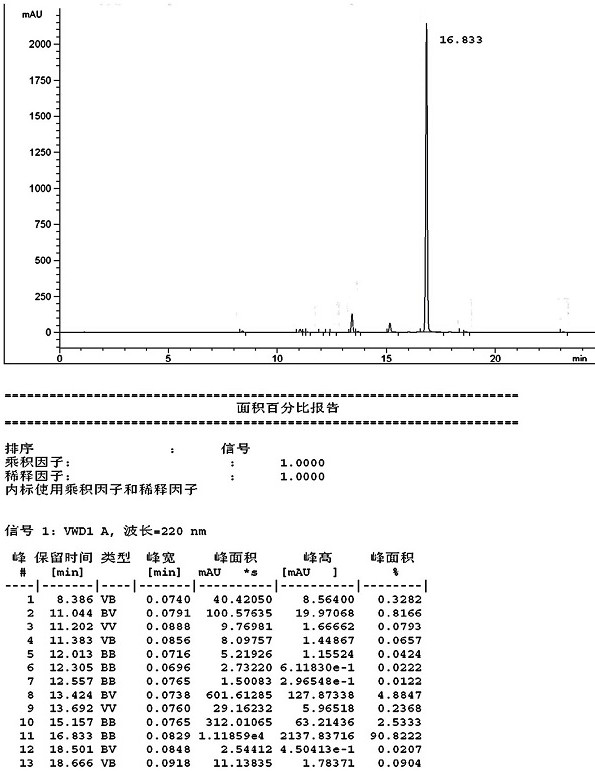
[0039] Add methyl 2-oxoindole-6-carboxylate (15g, 78.5mmol), triethyl orthobenzoate (49.5g, 220.7mmol) and acetic anhydride (150ml, 1.59mol) into the reaction flask in turn, and heat to 110°C, heat preservation reaction for 4 hours, cooling down, solids precipitated, filtered, and vacuum dried at 50°C for 16 hours to obtain 22g, with a mass yield of 146.7% and a molar yield of 76.8%. HPLC purity 93.35%*, impurity 1 0.82%, impurity 2 4.9%.
[0040] NMR data: 1 H-NMR: δ1.35(t, 3H), 2.44 (s, 3H), 3.87(s, 3H), 3.98-4.03 (q, 2H), 7.52-7.57 (m, 5H), 7.87-7.89(d , 1H), 8.07-8.09(d, 1H), 8.73(s, 1H).
[0041] Wherein, the HPLC detection spectrum of the formula III compound that embodiment 1 prepares is as follows figure 1 shown.
Embodiment 2
[0042] Example 2 Preparation of 1-acetyl-3-(ethoxy(phenyl)methylene)-2-oxoindoline-6-carboxylic acid methyl ester
[0043] Methyl 2-oxoindole-6-carboxylate (20g, 104.6mmol), triethyl orthobenzoate (46.9g, 209.2mmol), acetic anhydride (32.0g, 313.8mmol) and 200mL xylene were added to the reaction in sequence In the bottle, heated to 110°C, kept the reaction for 4 hours, cooled to room temperature, filtered, and vacuum dried at 50°C for 16 hours to obtain 30.9g, mass yield 154.5%, molar yield 80.9%, HPLC purity 99.43%*, impurity 1 0.03 %, impurity 2 0.03%.
[0044] Analysis through structural confirmation shows that the resulting product is identical to 1-acetyl-3-(ethoxyl (phenyl) methylene)-2-oxoindoline-6-formic acid methyl ester in Example 1 substance.
Embodiment 3
[0045] Example 3 Preparation of 1-acetyl-3-(ethoxy(phenyl)methylene)-2-oxoindoline-6-carboxylic acid methyl ester
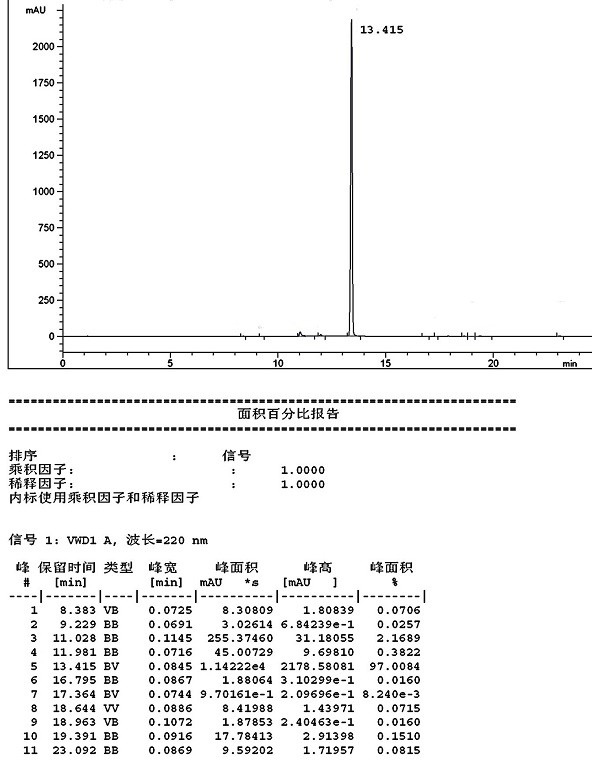
[0046]Methyl 2-oxoindole-6-carboxylate (20g, 104.6mmol), triethyl orthobenzoate (46.9g, 209.2mmol), acetic anhydride (32.0g, 313.8mmol) and xylene 200mL were added to the reaction in sequence In the bottle, heated to 120°C, kept for reaction for 4 hours, cooled to room temperature, filtered, and vacuum-dried at 50°C for 16 hours to obtain 31.5g, mass yield 157.5%, molar yield 82.4%, HPLC purity 99.75%*, impurity 1 not Detected, impurity 2 <0.01%.
[0047] Analysis through structural confirmation shows that the product obtained is identical to 1-acetyl-3-(ethoxyl (phenyl) methylene)-2-oxoindoline-6-formic acid methyl ester in Example 1 substance.
[0048] Wherein, the HPLC detection spectrum of the formula III compound that embodiment 3 prepares is as follows Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com