Infectious medical waste disinfectant and preparation method thereof
A technology of medical waste and disinfectant, applied in botany equipment and methods, disinfectants, chemicals for biological control, etc., can solve the problems of narrow disposal range, poor stability, difficulties, etc., and improve the ability of sterilization and disinfection , Improve the bonding strength and improve the effect of bactericidal ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
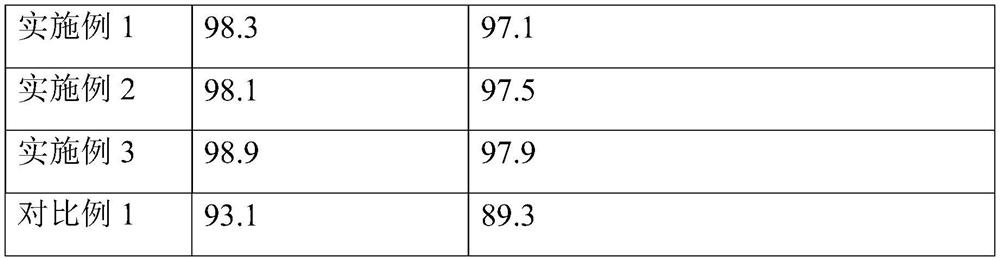
Embodiment 1
[0026] A kind of infectious medical waste disinfectant of the present embodiment comprises the raw material of following weight portion:
[0027] 55 parts of modified nano zinc oxide, 15 parts of sodium camphorsulfonate, 10 parts of antibacterial stabilizer, 3 parts of hydrogen peroxide, 20 parts of deionized water;
[0028] The preparation method of described antibacterial stabilizer is:
[0029] Step 1: Hydrotalcite modification: crush the hydrotalcite into particles with a particle size of 1mm, put it in a water bath at a temperature of 70°C for 11 minutes, and then send it to a calciner for calcination, with a temperature increase of 1.5°C / min Raise the temperature to 150°C, hold for 10 minutes, then anneal to 90°C, continue to hold for 15 minutes, and finally cool down to room temperature naturally;
[0030] Step 2: Antibacterial hydrotalcite: Add hydrotalcite to the fungicide and stir at a speed of 100r / min for 20 minutes, then add starch additives, and then continue st...
Embodiment 2
[0039] A kind of infectious medical waste disinfectant of the present embodiment comprises the raw material of following weight portion:
[0040] 65 parts of modified nano zinc oxide, 25 parts of sodium camphorsulfonate, 20 parts of antibacterial stabilizer, 5 parts of hydrogen peroxide, 30 parts of deionized water;
[0041] The preparation method of described antibacterial stabilizer is:
[0042] Step 1: Hydrotalcite modification: crush the hydrotalcite into granules with a particle size of 2 mm, put it in a water bath at 80°C for 13 minutes, and then send it into a calciner for calcination, with a heating rate of 2.5°C / min Raise the temperature to 158°C, hold for 20 minutes, then anneal to 110°C, continue to hold for 25 minutes, and finally cool down to room temperature naturally;
[0043] Step 2: Antibacterial hydrotalcite: Add hydrotalcite to the fungicide and stir at a speed of 200r / min for 30 minutes, then add starch additives, and then continue stirring for 25 minutes....
Embodiment 3
[0052] A kind of infectious medical waste disinfectant of the present embodiment comprises the raw material of following weight portion:
[0053] 60 parts of modified nano zinc oxide, 20 parts of sodium camphorsulfonate, 15 parts of antibacterial stabilizer, 4 parts of hydrogen peroxide, 25 parts of deionized water;
[0054] The preparation method of described antibacterial stabilizer is:
[0055] Step 1: Hydrotalcite modification: crush the hydrotalcite into particles with a particle size of 1-2mm, put it in a water bath at a temperature of 75°C for 12 minutes, and then send it to a calciner for calcination at a rate of 2.0°C / min The heating rate is raised to 154°C, kept for 15 minutes, then annealed to 100°C, kept for 20 minutes, and finally cooled to room temperature naturally;
[0056] Step 2: Antibacterial hydrotalcite: Add hydrotalcite to the fungicide and stir at a speed of 150r / min for 25 minutes, then add starch additives, then continue stirring for 20 minutes, after...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com