Medicine with imaging and metastatic bone tumor treatment functions as well as preparation method and application of medicine
A bone tumor and metastatic technology, applied in the field of nuclear medicine, can solve the problem of low action intensity, and achieve the effects of short reaction time, simple method and scientific design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
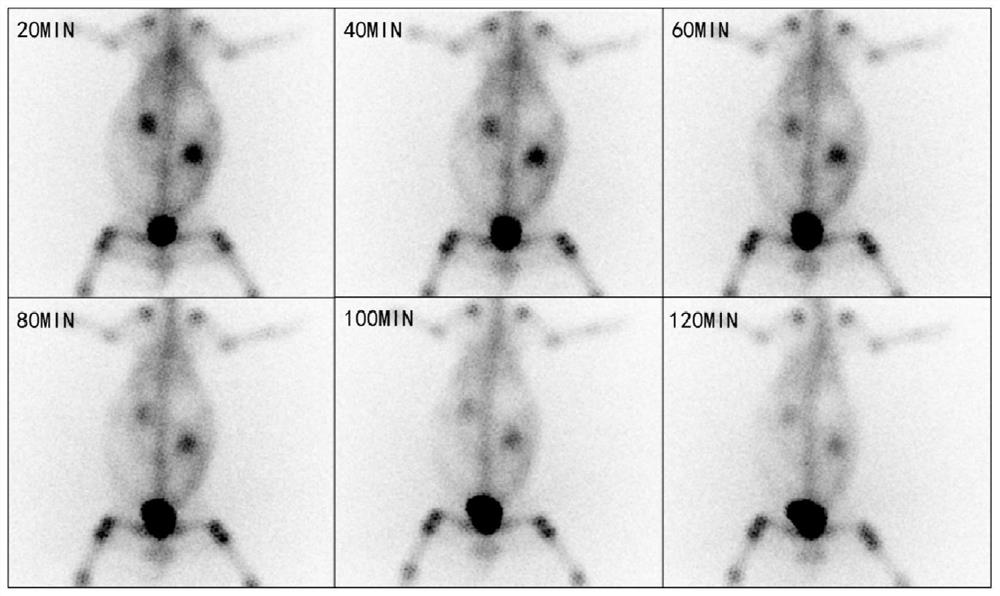
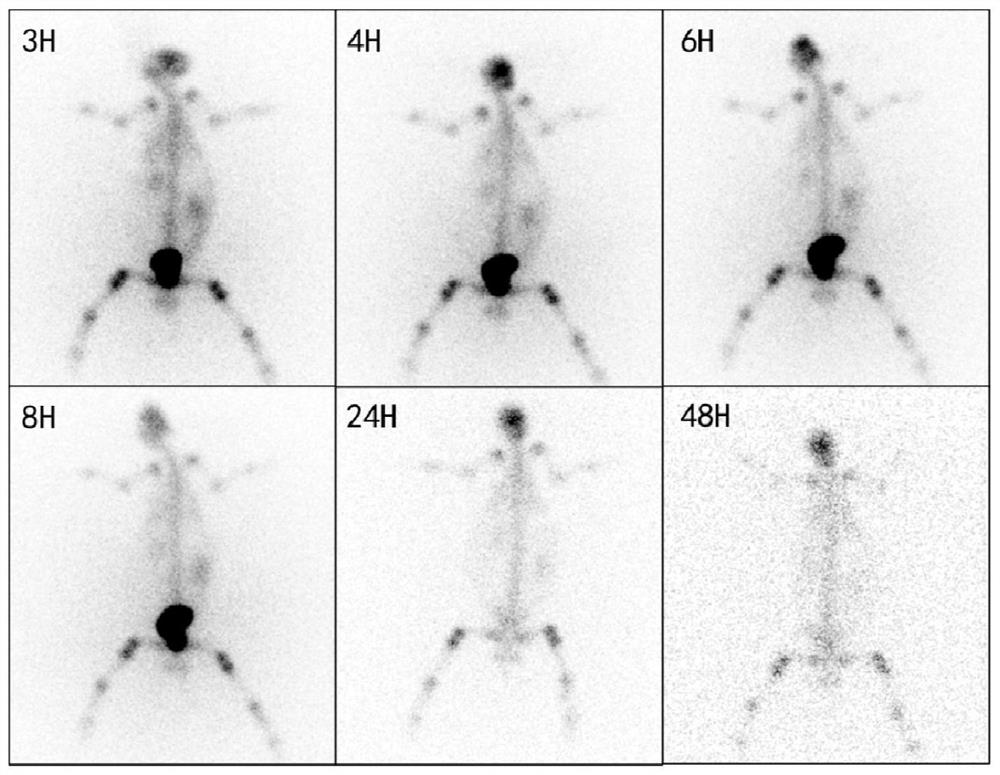
Image
Examples
Embodiment 1
[0027] Present embodiment 1 has investigated the consumption of stannous chloride among the present invention, specifically:
[0028] Take 15 EP tubes, add ibandronic acid (30mg / ml) 1.2mg, ascorbic acid (50mg / ml) 0.3mg to each tube in turn, and then add stannous chloride solution (0.1 molar hydrochloric acid to prepare 6mg / ml) 0.02mg, 0.04mg, 0.06mg, 0.08mg, 0.10mg, 0.12mg, 0.14mg, 0.16mg, 0.18mg, 0.20mg, 0.24mg, 0.28mg, 0.32mg, 0.36mg, 0.40mg, then add Potassium perrhenate (0.2mg / ml) 0.005mg, freshly rinsed Na 188 ReO 4 The eluent (370MBq / ml) is 37MBq, adjust the pH value to about 2 with 1 mole of sodium acetate solution and 1 mole of hydrochloric acid, shake and mix well, react in a metal bath at 95°C for 30min, after the reaction is completed and cool to room temperature, The pH value of the tube is adjusted to 6-7, and a 0.22 μm filter membrane is used for sterilization and filtration. Then it was determined by thin layer paper chromatography (TLC) 188 The radiochemic...
Embodiment 2
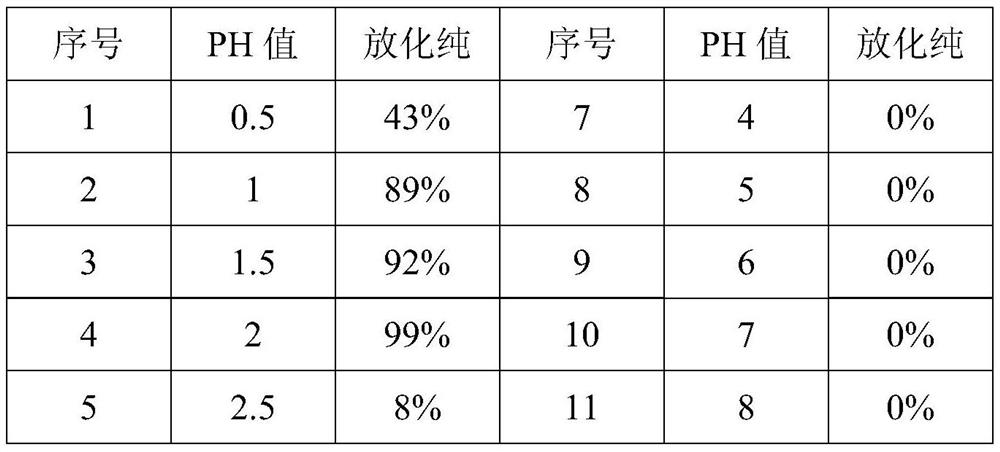
[0033] The present embodiment investigates the pH value among the present invention, specifically:
[0034] Take 12 EP tubes, add 1.2 mg of ibandronic acid (30 mg / ml), 0.3 mg of ascorbic acid (50 mg / ml), 0.14 mg of stannous chloride (6 mg / ml with 0.1 mole of hydrochloric acid) to each tube in turn, Potassium perrhenate (0.2mg / ml) 0.005mg, freshly rinsed Na 188 ReO 4Eluate (370MBq / ml) 37MBq, adjust the pH value to 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 7, 8, 9 with 1 mole of sodium acetate solution and 1 mole of hydrochloric acid , shake and mix well, and react in a metal bath at 95°C for 30 minutes. After the reaction is completed and cooled to room temperature, adjust the pH value of each tube to 6-7, and use a 0.22 μm filter membrane to sterilize and filter. Then it was determined by thin layer paper chromatography (TLC) 188 The radiochemical purity of Re-ibandronic acid, the results are shown in Table 2:
[0035] Table 2 The results of radiochemical purity under different pH ...
Embodiment 3
[0040] The present embodiment investigates the dosage of ibandronic acid in the present invention, specifically:
[0041] Take 15 EP tubes, add ibandronic acid (30mg / ml) 0.2mg, 0.4mg, 0.6mg, 0.8mg, 1.0mg, 1.2mg, 1.4mg, 1.6mg, 1.8mg, 2.0mg, 2.4 mg, 2.8mg, 3.2mg, 3.6mg, 4.0mg, then add ascorbic acid (50mg / ml) 0.3mg, stannous chloride (0.1 molar hydrochloric acid to 6mg / ml) 0.14mg, potassium perrhenate (0.2mg / ml) 0.005mg, freshly rinsed Na 188 ReO 4 The eluent (370MBq / ml) is 37MBq, adjust the pH value to about 2 with 1 mole of sodium acetate solution and 1 mole of hydrochloric acid, shake and mix well, react at 95°C for 30min, after the reaction is completed and cool to room temperature, adjust each tube The pH value is 6-7, and a 0.22 μm filter membrane is used for sterilization and filtration. Then it was determined by thin layer paper chromatography (TLC) 188 The radiochemical purity of Re-ibandronic acid, the results are shown in Table 3 below:
[0042] Table 3 The resul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com