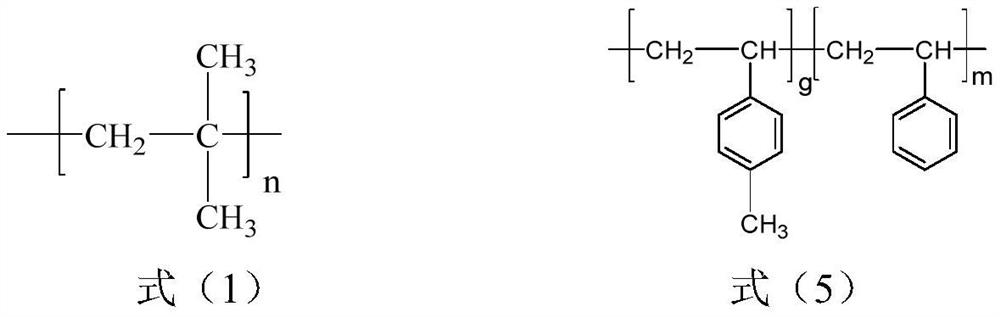A kind of styrenic block copolymer and preparation method thereof
A technology of block copolymers and styrenes, applied in the field of styrenic block copolymers and their preparation, can solve the problems of difficult control of sulfonation reaction process, complex monomer structure, cumbersome steps of hydroxyl functionalized polymers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of the styrenic block copolymer provided by the invention comprises the following steps:
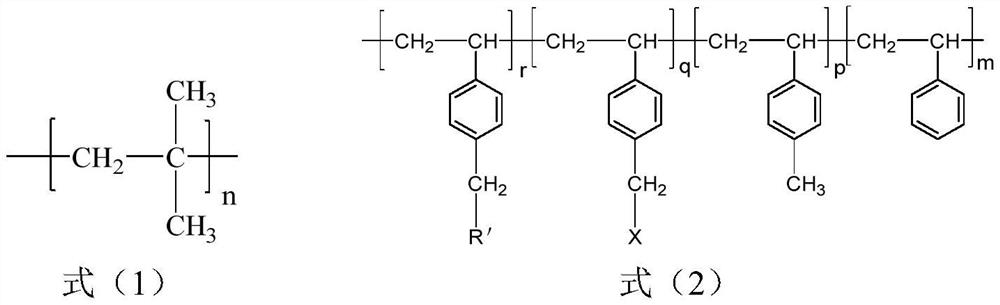
[0028] S1, isobutylene, styrene and p-methylstyrene are obtained through sequential living cationic copolymerization in the presence of an initiating system to obtain a polyisobutylene segment with a structure shown in formula (1) and styrene with a structure shown in formula (5) Block copolymers (SMB) of random copolymerized segments with p-methylstyrene structural units;
[0029]
[0030] Wherein, n is 200~1800, the total number (m) of styrene structural units accounts for 0.5~29% of the total moles of all structural units, and the total number (g) of p-methylstyrene structural units accounts for 0.5~29% of the total moles of all structural units. 1.05-43%.
[0031] S2, carry out bromination reaction / chlorination reaction in organic solvent with the block copolymer obtained in step S1 and bromination / chlorination reagent, obtain bromination / chlorinatio...
Embodiment 1
[0078] This example serves to illustrate the azonia-ionized poly[(p-methylstyrene-co-styrene)-b-isobutylene-b-(styrene-co-p-methylstyrene)] block copolymer (FSMB -1) Preparation and properties.
[0079] Preparation of S1 and SMB-1
[0080] Under the protection of high-purity nitrogen at -78°C, 110 mL of dichloromethane, 164 mL of n-hexane, 0.3 mol of isobutene (IB) and 0.845 mmol of bifunctional initiator 1,4-bis(2-chloro-2 -Propyl)benzene (DCC), add 7.0mL containing co-initiator FeCl 3 With the additive isopropanol (POH) solution (of which: FeCl 3 :POH=1:1.4), so that the concentration of IB in the reaction system is 1.0mol / L, DCC:FeCl 3 : IB=1:3.6:355 (molar ratio), reaction 15min, IB monomer is fully converted, obtains polyisobutylene double-end active chain; Add 100mL containing styrene (St, 68.7mmol) and p-methylstyrene (pMS, 34.4mmol) in dichloromethane / normal hexane (4 / 6, v / v) mixed solution, further carry out active cationic block copolymerization reaction, the rea...
Embodiment 2
[0088] The preparation method of S1 and SMB-2 is the same as step S1 in Example 1. Under the protection of high-purity nitrogen at -80°C, add 220mL of dichloromethane, 328mL of n-hexane, 0.6mol of isobutene and 3.38mmol of DCC into the polymerization reactor and mix well, add 14mL of FeCl containing co-initiator 3 With POH solution (where: FeCl 3 :POH=1:1.4), so that the concentration of IB in the reaction system is 1.0mol / L, DCC:FeCl 3 : IB=1:3.6:178 (molar ratio), polymerization reaction 15min, IB monomer is fully transformed, obtains polyisobutylene double-end active chain; Only the feeding amount of St and pMS mixed solution is reduced, add 120mL containing 82.4mmol styrene and 41.2mmol methylene chloride / n-hexane (4 / 6, v / v) mixed solution of p-methylstyrene, obtain poly(styrene-co-p-methylstyrene)-b-polyisobutylene-b-poly( Styrene-co-p-methylstyrene) triblock copolymer SMB-2.
[0089] S2, BSMB-2 preparation method is the same as step S2 in embodiment 1, just Br2 The am...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| storage modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com