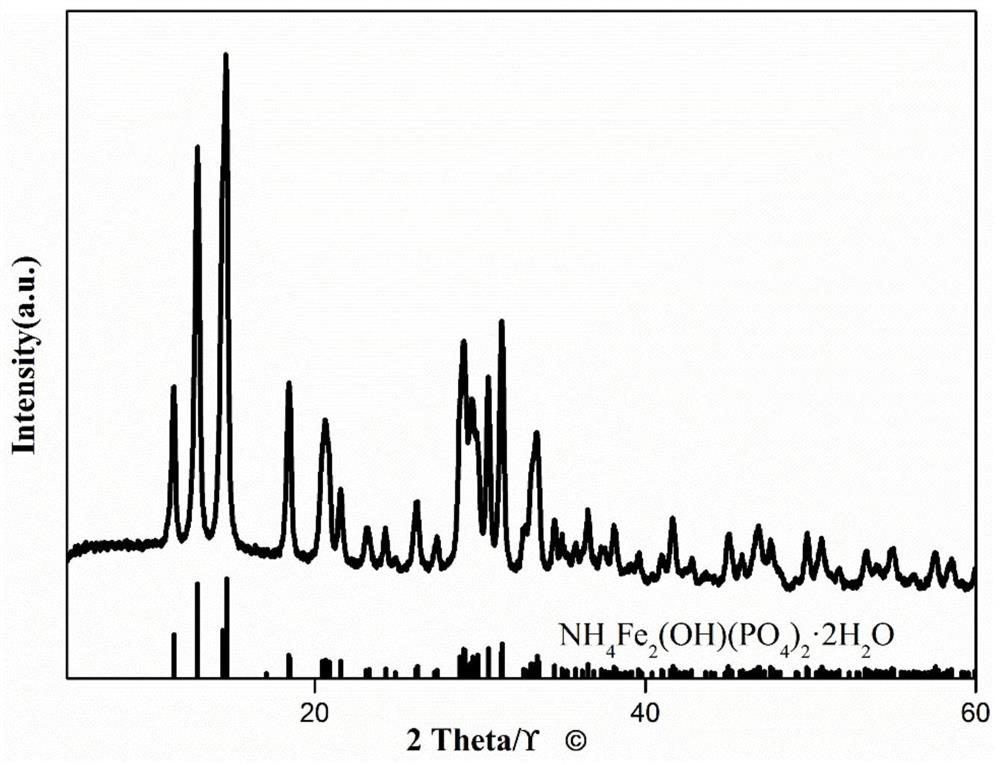
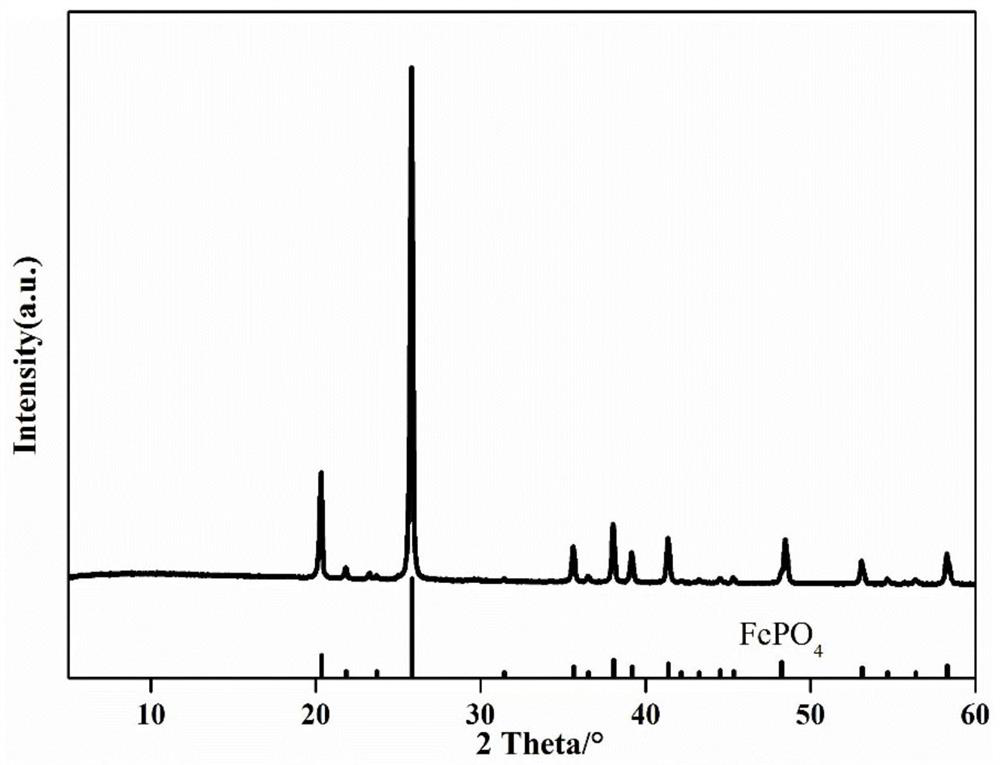
Preparation method and application of iron phosphate
A technology of ferric phosphate and ammonium ferric phosphate, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of increasing production cost, increasing production cost, consumption price, etc. The effect of high solid density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The preparation method of the iron phosphate of the present embodiment specifically comprises the following steps:
[0054] (1) the waste iron phosphate dihydrate of 50kg is roasted at 350 DEG C for 3h to remove crystal water, the material after roasting is about 40kg, and the material is dropped into the sulfuric acid solution kettle storing 270L, 1.5mol / L and stirred at 200rpm rotating speed, Heated to 50°C to dissolve for about 5 hours, and then left to stand. The filter residue was filtered out by a precision filter and then transferred to a storage tank to obtain Fe-containing 3+ and PO4 3- solution, the iron and phosphorus content in the detection solution were 43.28g / L and 24.78g / L respectively, and the molar ratio of Fe:P=1:1.03;
[0055] (2) 50L deionized water is the bottom liquid, which will contain Fe 3+ and PO4 3- The solution and ammonia water are injected into the reaction kettle in parallel from the bottom of the reactor at a 6:1 liquid feed rate, adj...
Embodiment 2
[0063] The preparation method of the iron phosphate of the present embodiment specifically comprises the following steps:
[0064] (1) the ferric phosphate waste material of 10kg is roasted at 400 DEG C for 5h to remove crystal water, the material after roasting is about 8kg, and at 200rpm rotating speed, the material is put into the sulfuric acid solution still storing 34L, 2.4mol / L and stirred, heated to After dissolving at 50 °C for about 5 hours, let it stand, filter out the filter residue with a precision filter, and transfer it to a storage tank to obtain Fe-containing 3+ and PO4 3- The iron and phosphorus content in the detection solution were 83.20g / L and 47.9g / L respectively, and the Fe:P molar ratio=1:1.04;
[0065] (2) 50L deionized water is the bottom liquid, which will contain Fe 3+ and PO4 3- The solution and ammonia water are injected into the reactor by the bottom of the reactor at a rate of 3:1, and the fine-tuning feeding speed of the lye is adjusted by th...
Embodiment 3
[0073] The preparation method of the battery-grade iron phosphate of the present embodiment specifically includes the following steps:
[0074] (1) the ferric phosphate waste material of 4kg is roasted at 300 DEG C for 3h to remove crystal water, the material after roasting is about 4kg, and the material is dropped into the sulfuric acid solution kettle that stores 27L, 1.5mol / L under 200rpm rotating speed and stirred, heated to After dissolving at 50 °C for about 5 hours, let it stand, filter out the filter residue with a precision filter, and transfer it to a storage tank to obtain Fe-containing 3+ and PO4 3- The iron and phosphorus content in the detection solution were 63.42g / L and 37.17g / L respectively, and the Fe:P molar ratio=1:1.05;
[0075] (2) 20L deionized water is used as the bottom liquid, which will contain Fe 3+ and PO4 3- The solution and ammonia water are injected into the reactor by the bottom of the reactor at a rate of 8:1, and the fine-tuning feeding sp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tap density | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Tap density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com