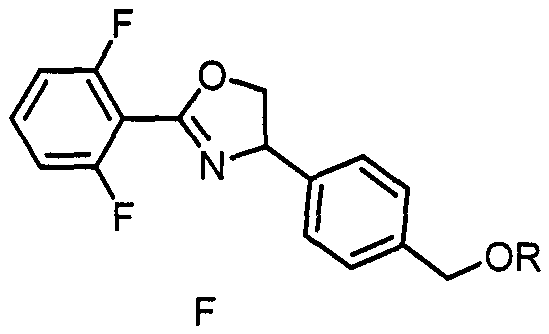
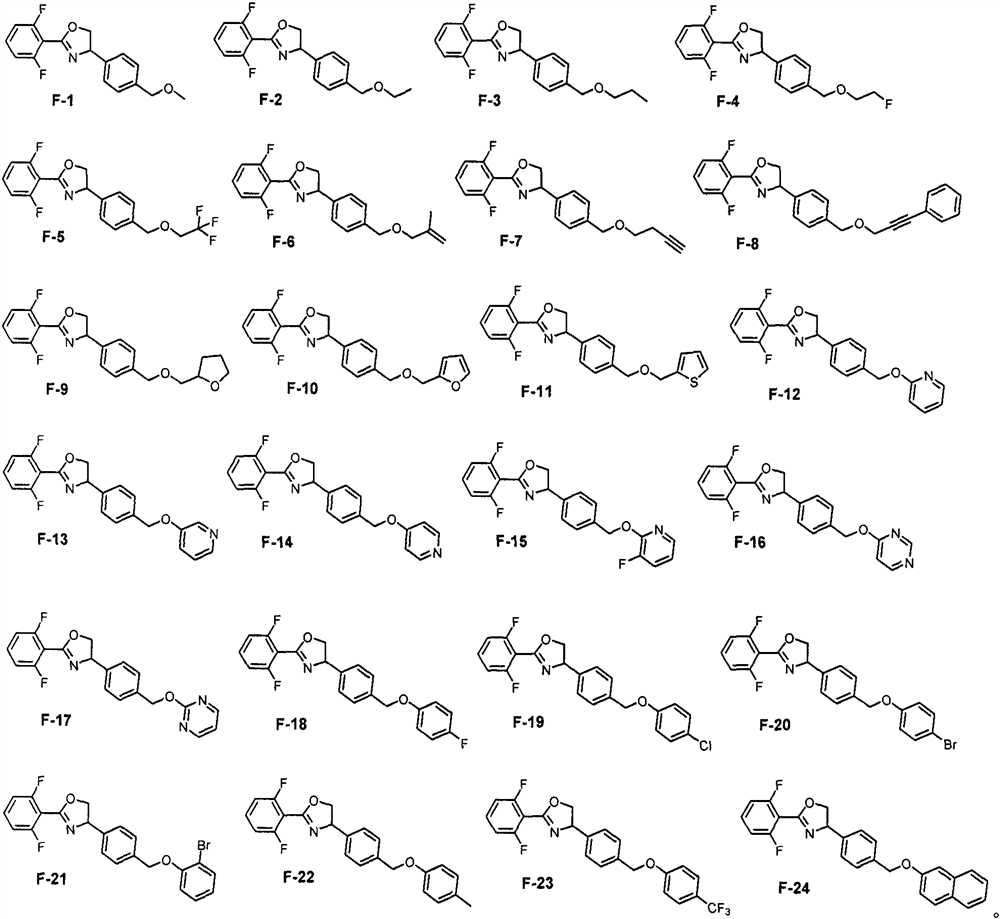
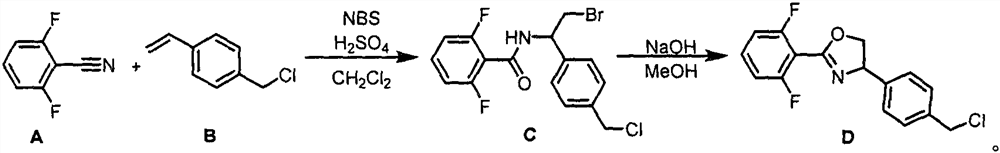
Ether bond-containing oxazoline derivative, design synthesis and application thereof in prevention and treatment of phytophagous mites
A technology of oxazolines and derivatives, applied in the directions of botanical equipment and methods, applications, acaricides, etc., can solve the problem that insects cannot form epidermis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the synthesis of C
[0024] Add A (15.3g, 110mmol), B (15.2g, 100mmol), NBS (19.6g, 110mmol), 125mL of CH 2 Cl 2 . Concentrated sulfuric acid (6.5 mL, 120 mmol) was slowly added dropwise to the system under ice-bath stirring, and the stirring was continued under ice-bath. The reaction was monitored by TLC (PE / EA=4 / 1), and the reaction was completed after stirring at room temperature for 8 hours. Dichloromethane and saturated sodium thiosulfate solution were added to separate the layers, and the aqueous phase was extracted three times with dichloromethane. After combining the organic phases, they were washed once with saturated sodium bicarbonate solution and saturated sodium chloride solution, dried and filtered over anhydrous sodium sulfate, and concentrated under reduced pressure to remove dichloromethane. After column chromatography with PE / EA=6 / 1, 33.4 g of light yellow solid was obtained, the yield was 86%, and the melting point was 128-129°C.
[...
Embodiment 2
[0026] Embodiment 2: the synthesis of D
[0027] The synthesized substrate C (19.4 g, 50 mmol) was added into a 100 mL single-necked bottle, and 30 mL of methanol was added to dissolve it. Sodium methoxide (2.97 g, 55 mmol) was added with stirring in an ice-water bath. Stir at room temperature. The reaction was monitored by TLC (PE / EA=4 / 1), and the reaction was completed after 5 min. Methanol was spun off, water and dichloromethane were added to separate the layers, and the aqueous phase was extracted three times with dichloromethane. The combined organic phases were washed once with water and once with saturated sodium chloride, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to remove dichloromethane. After spin-drying, 12.8 g of light yellow solid D was obtained, with a yield of 83%. The melting point is 64-65°C.
[0028] 1 H NMR (400MHz, DMSO-d 6 )δ7.73-7.62(m, 1H), 7.47(d, J=7.8Hz, 2H), 7.34(d, J=8.0Hz, 2H), 7.30(t, J=8.4Hz, 2...
Embodiment 3
[0029] Embodiment 3: F-1, F-2, the synthesis of F-3
[0030] Substrate D (0.31g, 1mmol), sodium alkoxide (0.51g, 1.0mmol) and 10mL dry THF were added to a 100mL single-necked flask to dissolve, and refluxed under the protection of argon. The reaction was monitored by TLC (PE / EA=4 / 1), and the reaction was completed after 2 hours. Water and dichloromethane were added to separate layers, the aqueous phase was extracted with dichloromethane 3 times, the combined organic phases were washed 2 times with water, washed 1 time with saturated sodium chloride, dried over anhydrous sodium sulfate, solvent extraction, column chromatography (PE / EA = 6 / 1).
[0031] Colorless transparent oily liquid, yield 67%. 1 H NMR (400MHz, DMSO-d 6 )δ7.76-7.61 (m, 1H), 7.44-7.21 (m, 6H), 5.50 (dd, J=10.0, 8.0Hz, 1H), 4.86 (dd, J=10.0, 8.8Hz, 1H), 4.42 (s, 2H), 4.20(t, J=8.4Hz, 1H), 3.30(s, 3H). 13 C NMR (100MHz, DMSO-d 6 )δ160.2 (dd, J=253.2, 6.1Hz), 155.8, 141.2, 137.6, 133.5, 127.8, 126.4, 112.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com