Preparation method of EBV specific cytotoxic T cells
A cytotoxic and specific technology, applied in the field of cell biology and biomedicine, can solve the problems of low efficiency, high cost and long time course of EBV-CTL preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
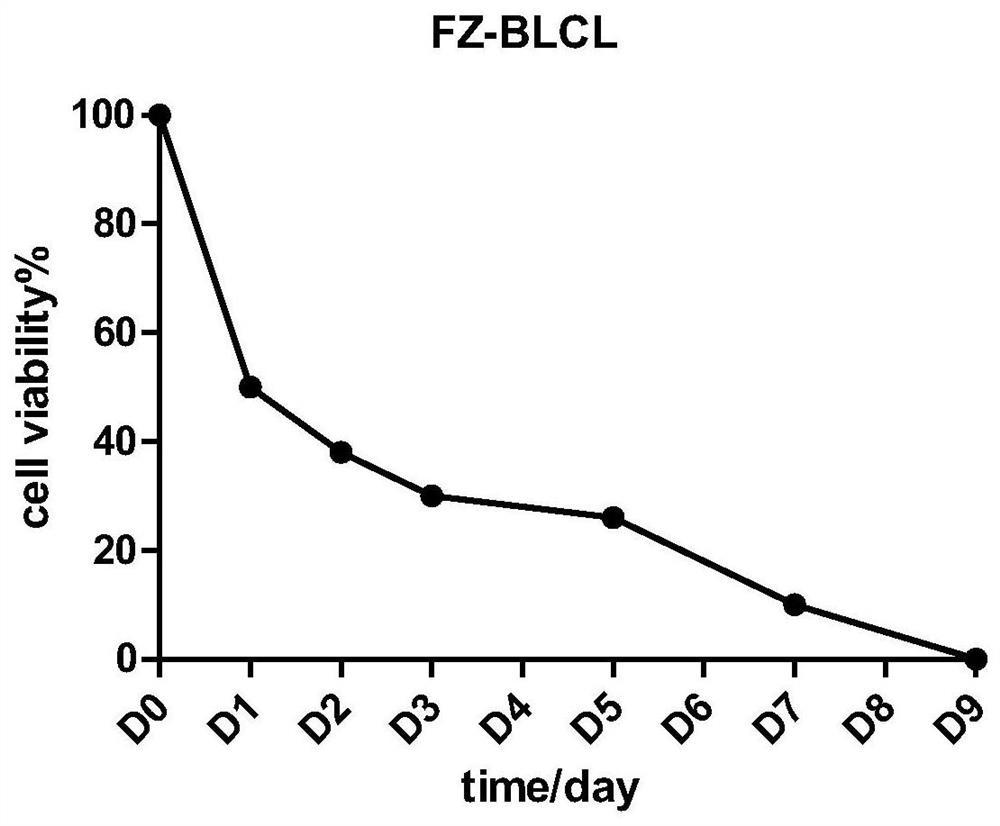
[0031] Example 1 Preparation of stimulated cell fz-BLCL
[0032] EBV-transformed human B lymphocytes can be obtained by infecting human B lymphocytes with viruses carrying EBV antigens. The EBV-transformed human B lymphocytes used in the present invention were purchased from the Kunming Cell Bank of the Type Culture Collection Committee of the Chinese Academy of Sciences (EBV-Human , No.: KCB 200546M, referred to as BLCL). The initial density of BLCL culture was 5.0×10 5 / mL, the culture medium used is RPMI-1640, add 20% FBS, add 1% Gluta Mix, pass once every two days during the culture process, BLCL culture condition is 37 ℃ incubator, containing 5% CO 2 and saturated water vapor. When the cells are expanded to a sufficient number, the reserved samples are frozen and irradiated:
[0033] The dose of BLCL cryopreservation is 1.0×10 7 / cartridge, the freezing solution is fetal bovine serum FBS containing DMSO at a final concentration of 10%, and the freezing volume is 1mL; ...
Embodiment 2
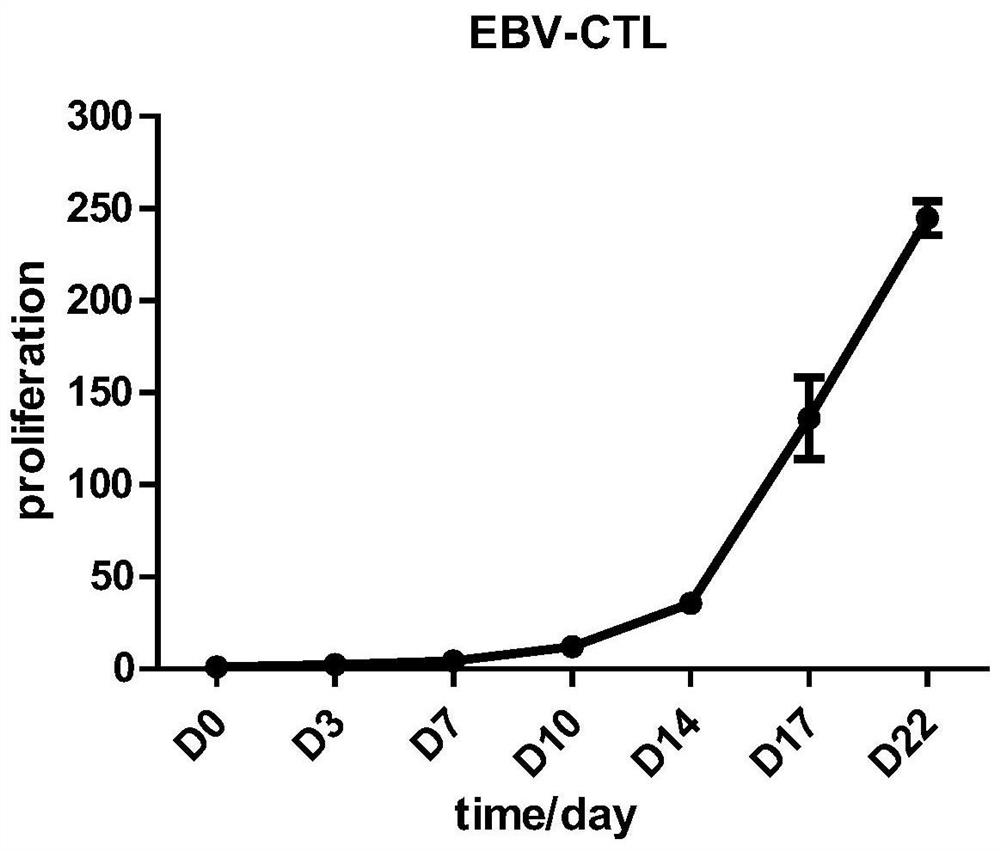
[0036] Example 2 Preparation, cultivation and identification of EBV-CTL
[0037] 1. Isolation of Mononuclear Cells from Cord Blood or Peripheral Blood
[0038] The source of the mononuclear cells is umbilical cord blood, and the isolation of the mononuclear cells is operated in a biological safety cabinet and obtained by the following methods:
[0039] (1) Divide the umbilical cord blood into 50mL centrifuge tubes, 45mL per tube, rise by 9 and fall by 7, centrifuge at 2000rpm for 10min;
[0040] (2) After centrifugation, remove the upper layer of plasma, dilute the blood cell suspension with normal saline at a ratio of 1:1, mix well, and slowly add the diluted blood cell suspension to the lymphocyte separation chamber at a ratio of 2:1. In a 50mL centrifuge tube of liquid, there is a clear interface layering, up 6 down 4, 400g, centrifuge for 20min;
[0041] (3) After centrifugation, the interface is divided into four layers, from top to bottom: supernatant layer, buffy coat...
Embodiment 3
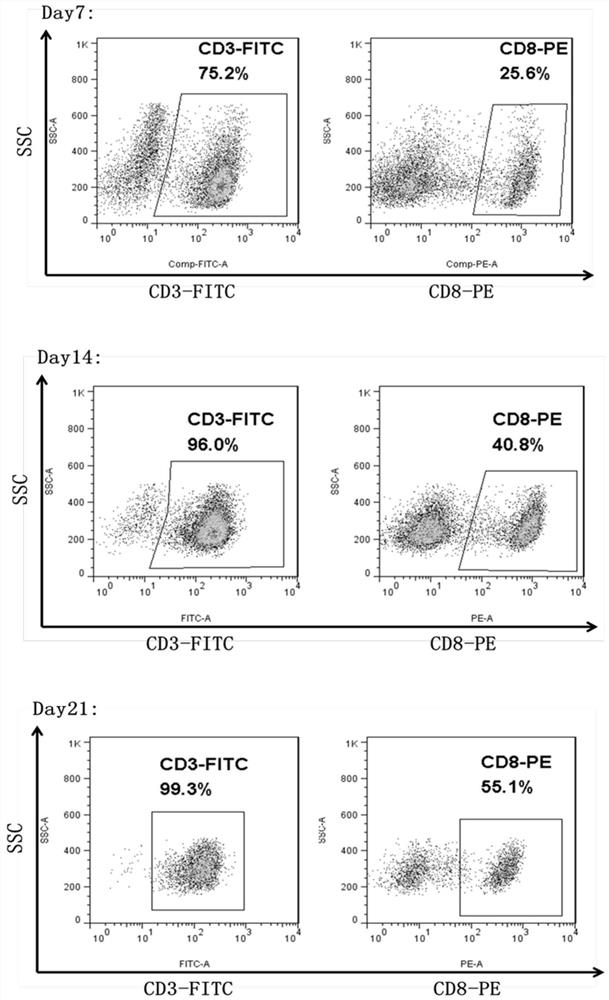
[0049] Example 3 In vitro killing efficiency of EBV-CTL
[0050] The EBV-CTL cultured to the 21st day in Example 2 was tested for cell-specific killing efficiency by an in vitro killing experiment. Two lymphocyte cell lines, BLCL and K562, were selected as target cells. Among them, the surface of BLCL cells expressed EBV antigen, while the surface of K562 cells did not express EBV antigen. The specificity of EBV-CTL was verified by detecting the apoptosis of BLCL and K562 cells Killing function:
[0051] BLCL and K562 were inoculated in a 96-well plate at a ratio of 1:1, and the T cells cultured to the 21st day, the EBV-CTL of Example 2 were inoculated according to the effect-to-target ratio E:T of 1:3 and 1:1, respectively. Cells were placed in 96-well plates, with 3 replicates per group, placed at 37°C, 5% CO 2 Cultured in an incubator for 24 hours, BLCL cells and K562 cells were detected by flow cytometry (such as Figure 5 shown), the antibodies used were PE anti-human ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com