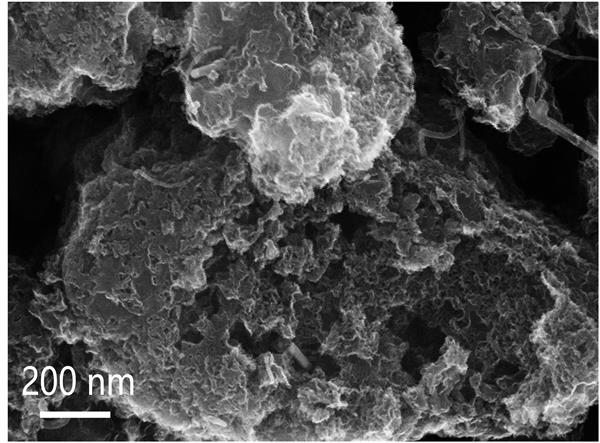
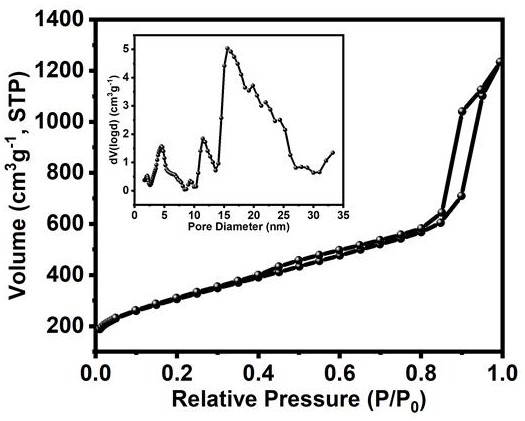
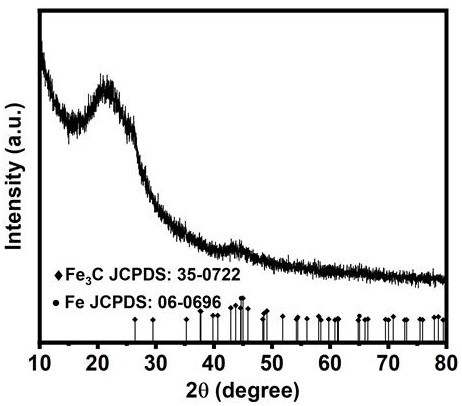
Preparation method of Fe/Fe3C nanoparticle-loaded porous nitrogen-doped carbon-based oxygen reduction catalyst
A nitrogen-doped carbon, nanoparticle technology, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve problems such as loss of catalytic active sites, achieve excellent oxygen reduction performance, accelerate material The effect of transmission, high effective specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Step S1: Dissolve 5g of melamine and 5mL of formaldehyde in 50mL of deionized water, place in a water bath at 60°C and stir for 30min to mix evenly, then add 1g of SiO 2 and 2 mL of glacial acetic acid, continued to stir at this temperature for 1 h, then stirred overnight at room temperature, centrifuged and dried to obtain material A1;
[0028]Step S2: Transfer material A1 to a nickel boat and place it in a tube furnace. In an inert gas atmosphere, first raise the temperature from room temperature to 300°C for 55 minutes and keep it for 60 minutes, then raise the temperature to 800°C at a heating rate of 5°C / min and Keep for 120min, then naturally cool to room temperature to obtain material B1;
[0029] Step S3: Transfer the material B1 to a 20 wt% hydrofluoric acid solution, soak it for 24 hours, wash it with high-purity water until neutral, and then dry it in an oven at 80°C for 12 hours to obtain the target product C1.
Embodiment 2
[0031] Step S1: Dissolve 5g of melamine and 5mL of formaldehyde in 50mL of deionized water, place in a water bath at 60°C and stir for 30min to mix evenly, then add 1g of SiO 2 , 0.5g iron acetylacetonate and 2mL glacial acetic acid, continue to stir at this temperature for 1h, then stir overnight at room temperature, obtain material A2 by centrifugation and drying:
[0032] Step S2: Transfer the material A2 to a nickel boat and place it in a tube furnace. In an inert gas atmosphere, first raise the temperature from room temperature to 300°C for 55 minutes and keep it for 60 minutes, then raise the temperature to 800°C at a heating rate of 5°C / min. Keep for 120min, then naturally cool to room temperature to obtain material B2;
[0033] Step S3: Transfer the material B2 to a 20 wt% hydrofluoric acid solution, soak it for 24 hours, wash it with high-purity water until neutral, and then dry it in an oven at 80°C for 12 hours to obtain the target product C2.
Embodiment 3
[0035] Step S1: Dissolve 5g of melamine and 5mL of formaldehyde in 50mL of deionized water, place in a water bath at 60°C and stir for 30min to mix evenly, then add 1g of SiO 2 , 0.7g iron acetylacetonate and 2mL glacial acetic acid, continue to stir at this temperature for 1h, then stir overnight at room temperature, obtain material A3 by centrifugation and drying:
[0036] Step S2: Transfer the material A3 to a nickel boat and place it in a tube furnace. In an inert gas atmosphere, first raise the temperature from room temperature to 300°C for 55 minutes and keep it for 60 minutes, then raise the temperature to 800°C at a heating rate of 5°C / min. Keep for 120min, then naturally cool to room temperature to obtain material B3;
[0037] Step S3: Transfer the material B3 into a 20 wt% hydrofluoric acid solution, soak it for 24 hours, wash it with high-purity water until neutral, and then dry it in an oven at 80°C for 12 hours to obtain the target product C3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com