Application of aescin in treatment of non-alcoholic fatty liver diseases
A kind of escin, non-alcoholic technology, applied in the application field of escin in the treatment of non-alcoholic fatty liver disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]Example 1: HepG2 cell culture
[0024]1 Medium-producing formulation: 10% FBS, 1% teeth, 4.5 g / L of DMEM medium of glucose;
[0025]2 inoculation: Turn HepG2 cells in 6-well plate, each hole density of 1 × 106Cells and placed them in a 37 ° C cell incubator containing 5% carbon dioxide and 95% air;
[0026]3 Hunger cells: After 12 h, the medium was removed, replaced with only 4.5 g / l glucose DMEM medium, hungry HepG2 cells for 3h;
[0027]4 Experimental group: HepG2 cells were incubated with 2 μm seven-leaf vessel (1000 μm free fatty acid (oleic acid: palmitic acid = 2: 1) co-incubated cell 24 h; divided into a blank group (DMEM medium), the model group (1000 μm free fatty acid ), The control group (2 μm seven-leaf saponin), the experimental group (2 μm seven-blasside + 1000 μm free fatty acid).
Embodiment 2
[0028]Example 2: AML12 cell culture
[0029]1 Medium production: 1ml, ITS LiquidMedia Ssupplement (Sigma, 13146) 1ml, 40 ng / l DEXAMETHASONE (Sigma, D4902-100MG), FBS (Gibeo) 10ML ;
[0030]2 Inoculated: AML12 cells were inoculated into the six-hole plate and placed in a cell incubator containing 5% carbon dioxide and 95% air;
[0031]3 Hunger cells: After 12 h, the medium was removed, replaced with DMEM / F12 medium, hungry AML12 cells for 3h;
[0032]4 Experimental group: incubation AML12 cells were incubated with 2 μm seven-leaf saponins and then 1000 μm free fatty acid (oleic acid: palmitic acid = 2: 1) co-incubated cell 24 h; divided into a blank group (DMEM / F12 medium), the model group (1000 μm) Free fatty acids), control group (2 μm seven-leaf saponin), experimental group (2 μm seven-blasside + 1000 μm free fatty acid).
Embodiment 3
[0033]Example 3: Determination of TG content in HepG2 cells
[0034]1 Collect cells, 1000 rpm / min centrifuge 10min, discard the supernatant, and sedate the cells;
[0035]2 Clean 1 ~ 2 times, 1000 rpm / min for 10 min, abandoned the supernatant;
[0036]3 cells are broken, add 0.2 ml of the like-phosphate buffer for homogenate, ice water bath (power 300W, 3 ~ 5S / time, interval 30s, repeating 3 ~ 5 times);
[0037]4 Preparation of homogenies without centrifugation directly according to the kit manufacturer's manual. The determination absorbance was performed using the enzyme standard.
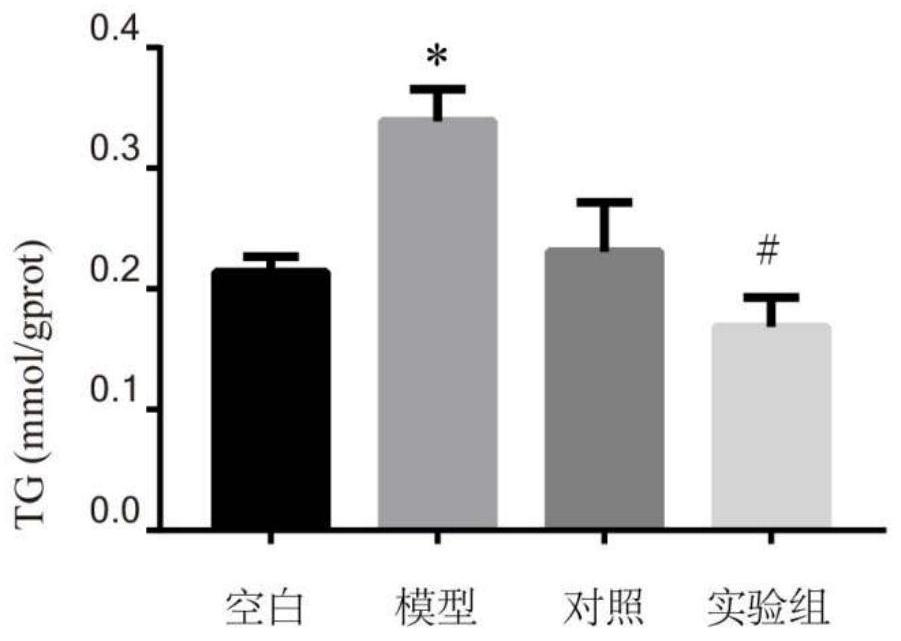
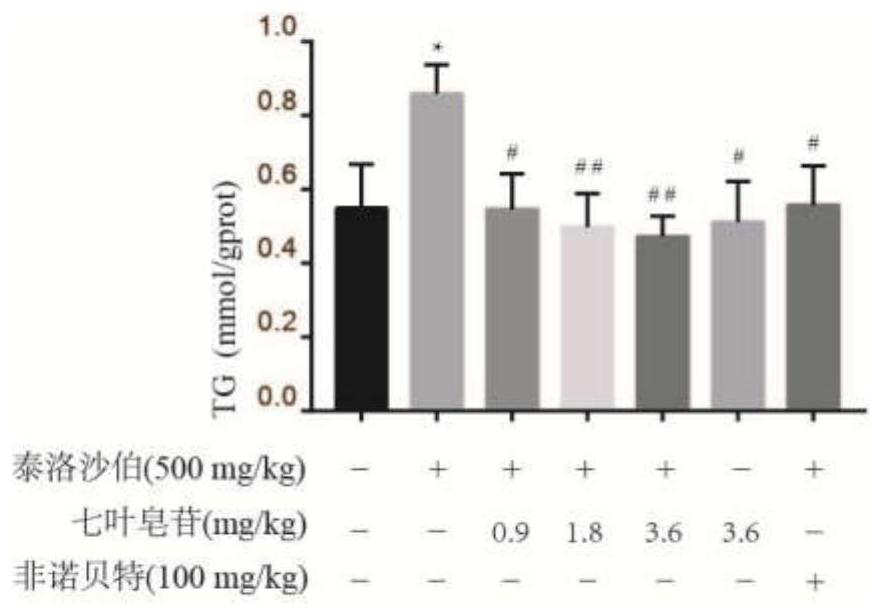
[0038]5 The TG content measured is attached.figure 1 . Attachfigure 1 The results showed that the free fatty acid of 1000 μm can significantly increase the Tg level in HepG2 cells as compared with the blank group. Compared with the model group, the seven-leaf saponin drug experiment can significantly reduce the Tg level in HepG2 cells (P<0.05).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com