Iron-based catalyst, preparation method and application thereof
A technology of catalysts and iron-based oxides, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxides/metal hydroxide catalysts, etc., can solve the problem of insufficient initial catalyst activity and achieve high activity , the effect of good technical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
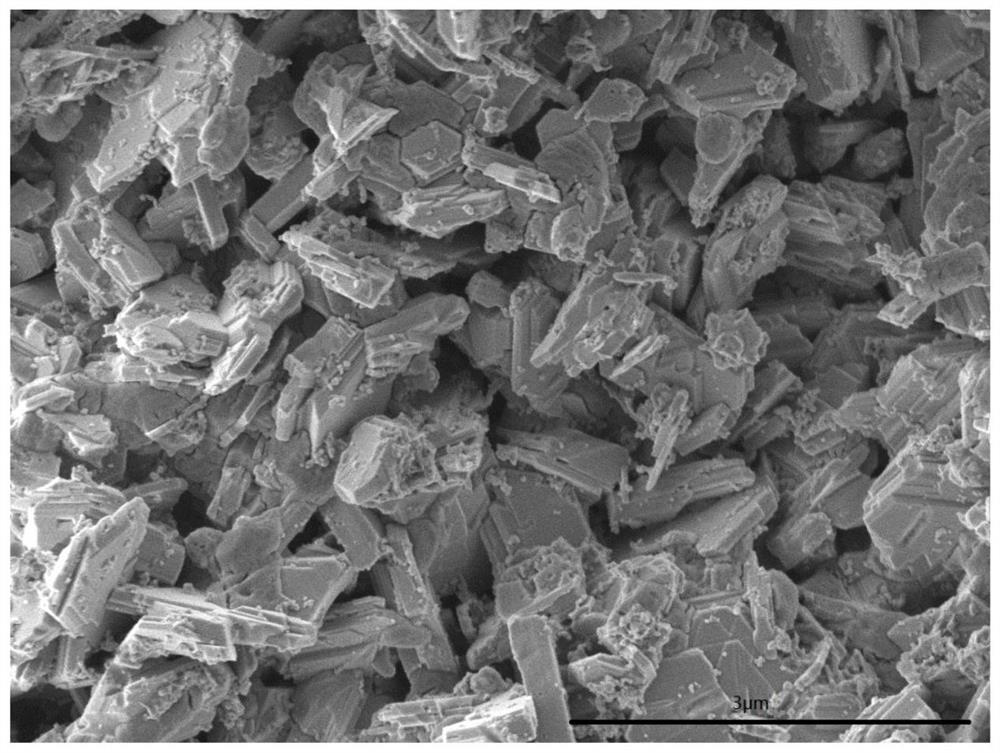
[0042] The preparation method of catalyst is to be equivalent to 48.39 parts of Fe 2 o 3 Iron oxide red, equivalent to 24.20 parts Fe 2 o 3 Iron oxide yellow, equivalent to 9.08 parts of CeO 2 The cerium oxalate was stirred in a kneader for 1 hour, and then the equivalent of 3.03 parts of CeO was added 2 The cerium nitrate aqueous solution was wet-kneaded for 0.6 hours, taken out, baked at 50°C for 3.5 hours, baked at 80°C for 10 hours, and then roasted at 500°C for 8 hours. After taking out, it was crushed into powder to obtain catalyst precursor I.
[0043] Catalyst precursor I, equivalent to 12.98 parts of K 2 Potassium carbonate of O, equivalent to 1.26 parts MoO 3Ammonium molybdate, calcium carbonate equivalent to 0.96 parts of CaO, 0.05 parts of MgO, 0.05 parts of ZnO and 5.0 parts of sodium carboxymethylcellulose were stirred in a kneader for 1.5 hours, and then an appropriate amount of deionized water was added for wet kneading for 0.6 hours. Take out the extrude...
Embodiment 2
[0063] The preparation method of catalyst is to be equivalent to 51.56 parts of Fe 2 o 3 iron oxide red, equivalent to 12.89 parts of Fe 2 o 3 iron oxide yellow, equivalent to 10.81 CeO 2 The cerium oxalate was stirred in a kneader for 1 hour, and then the equivalent of 5.03 parts of CeO was added 2 The cerium nitrate aqueous solution was wet-kneaded for 0.5 hours, taken out, baked at 60°C for 4.5 hours, baked at 90°C for 10 hours, and then roasted at 650°C for 5 hours. After taking out, it was crushed into powder to obtain catalyst precursor I.
[0064] Catalyst precursor I, equivalent to 13.40 parts K 2 Potassium carbonate of O, equivalent to 3.75 parts MoO 3 Ammonium molybdate, calcium carbonate equivalent to 2.45 parts of CaO, 0.12 parts of MgO and 5.0 parts of sodium carboxymethylcellulose were stirred in a kneader for 1.1 hours, and then an appropriate amount of deionized water was added for wet kneading for 0.6 hours, and the extrusion was taken out. Extrude into ...
Embodiment 3
[0067] The preparation method of catalyst is to be equivalent to 63.99 parts of Fe 2 o 3 Iron oxide red, equivalent to 16.00 parts Fe 2 o 3 iron oxide yellow, equivalent to 6.25 CeO2 The cerium oxalate was stirred in a kneader for 1 hour, and then calcined at 350° C. for 10 hours to obtain catalyst precursor I.
[0068] Catalyst precursor I, equivalent to 9.17 parts K 2 Potassium carbonate of O, equivalent to 0.77 parts MoO 3 Ammonium molybdate, calcium carbonate equivalent to 1.75 parts of CaO, 2.07 parts of MgO and 5.5 parts of sodium carboxymethylcellulose were stirred in a kneader for 1.5 hours, and then an appropriate amount of deionized water was added for wet kneading for 0.8 hours, and the extrusion was taken out. Extrude into particles with a diameter of 3 mm and a length of 5 mm, put them into an oven, bake at 80°C for 1.5 hours, bake at 120°C for 8 hours, then roast at 600°C for 6 hours, and then roast at 820°C for 5 hours to obtain a finished catalyst. The com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com