Saturated alkane dehydrogenation method
A technology for alkane and dehydrogenation, which is applied in the field of C3~C7 alkane dehydrogenation to olefins. It can solve the problems affecting the overall activity of the catalyst and achieve the effects of inhibiting the formation of SnPt alloy, improving utilization rate and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The catalyst precursor was prepared in the same manner as in Comparative Example 1. Then the catalyst is impregnated with a sulfurizing agent amount of 120% of the theoretical sulfur requirement of the dehydrogenation catalyst active metal, and the sulfurizing agent is ammonium sulfide, and dried (air atmosphere) at 120° C. for 4 hours to obtain a presulfided dehydrogenation catalyst. a.
[0048] Add 250mL of 0.005 mol / L chloroplatinic acid ethylene glycol solution to 48g of Sn-containing alumina carrier (dry basis), stir, and heat to reflux for 2h at a reflux temperature of 80°C. Then slowly add 32mL of 0.4 mol / L KOH ethylene glycol solution into the above system, raise the reflux temperature to 180°C, heat to reflux for 30min, and then cool to room temperature. The solution was filtered off and washed with deionized water until there was no chloride ion in the filtrate. Vacuum-dried at 120°C for 4h, then calcined at 500°C for 4h under nitrogen atmosphere. The resul...
Embodiment 2
[0052] The catalyst A prepared in Example 1 and the dehydrogenation catalyst B prepared in Example 1 were used.
[0053] The upper end of the dehydrogenation reactor is filled with catalyst A, and the lower end is filled with catalyst B. Wherein, the packing volume ratio of catalyst A and catalyst B is 1.5: 1.
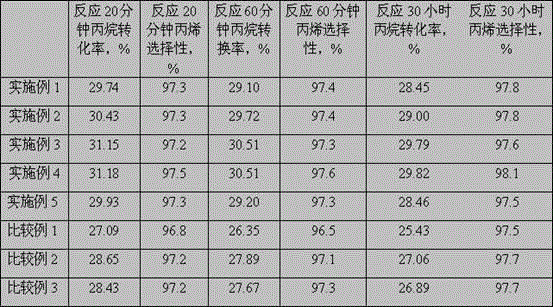
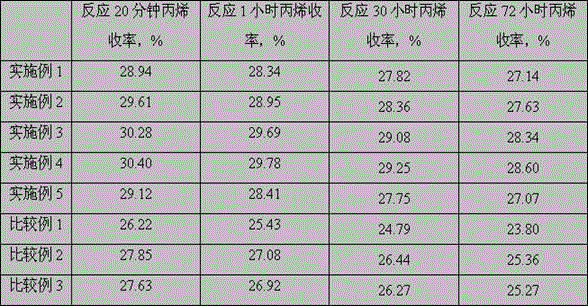
[0054] The reaction conditions are: volumetric space velocity 3000 h -1 , the reaction pressure is 0.1MPa, the reaction temperature is 600°C, and the molar ratio of hydrogen:propane is 1:1. Catalyst evaluation results are shown in Table 1 and Table 2.
Embodiment 3
[0056] Catalyst A prepared in Example 1 was used.
[0057] Add 300mL of 0.005 mol / L chloroplatinic acid ethylene glycol solution to 48g of Sn-containing alumina carrier (dry basis), stir, and heat to reflux for 2h at a reflux temperature of 80°C. Then slowly add 75mL of 0.4 mol / L KOH ethylene glycol solution into the above system, raise the reflux temperature to 180°C, heat to reflux for 30min, and then cool to room temperature. The solution was filtered off and washed with deionized water until there was no chloride ion in the filtrate. Vacuum-dried at 120°C for 4h, then calcined at 500°C for 4h under nitrogen atmosphere. The resulting solid, used at 70 °C containing KNO 3 Immerse in aqueous solution for 2 hours, vacuum dry at 120°C for 4 hours, and then bake at 500°C for 4 hours under nitrogen atmosphere. Catalyst C is prepared. The content of each component in the prepared catalyst is: Sn 0.3 wt%, Pt 0.6 wt%, K 1.0 wt%.
[0058] The upper end of the dehydrogenation rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com